Translate this page into:
Sperm microencapsulation in bovine: An overview

*Corresponding author: Nilendu Paul, Department of Veterinary Gynaecology and Obstetrics, Central Institute for Research on Buffaloes, Nabha, Punjab, India. nilendu364@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Paul N, Talluri TR, Kumaresan A. Sperm microencapsulation in bovine: An overview. J Reprod Healthc Med. 2024;5:7. doi: 10.25259/JRHM_14_2024
Abstract
Artificial insemination is so far the most successful and widely adopted assisted reproductive technique for genetic improvement in bovines. Despite their widespread adoption, the field conception rate using frozen semen straws remain low. Prediction of ovulation time, the short lifespan of frozen-thawed sperm, and retrograde backflow of semen following insemination result in fewer sperm available in the sperm reservoir in the oviduct and thus lessen the chances of fertilization. To tackle this, the idea of sustained release of spermatozoa inside the oviduct to maximize the odds of fertilizing the descendent ova is generated. Cell microencapsulation is a strategy that allows the implantation of cells, keeping the cells isolated from the host immune response by semipermeable membrane permitting the selective diffusion of gases, nutrients, and therapeutics but not of host immune cells. At present, few studies have been conducted where spermatozoa were encapsulated in a polymer shell/bead and successfully cryopreserved. The frozen-thawed encapsulated spermatozoa had shown comparable results in terms of in vitro functional assessment as well as in achieving pregnancy as compared to conventional semen dose. The purity of encapsulating material is very crucial to nullify maternal immune response as well as to achieve higher biocompatibility. In this manuscript, an overview of sperm encapsulation has been compiled, with more stress on types of encapsulating materials, their characterization, purity, and prospects of this method to come up as a robust method for achieving higher success following artificial insemination.
Keywords
Microencapsulation
Alginate
Fertility
Artificial insemination
Spermatozoa
INTRODUCTION
In a breeding program, the quality of semen plays a key role in deciding the establishment of pregnancy in females. A morphologically sound and functionally competent spermatozoa is highly desirable to fertilize ova successfully. Over the past few decades, the quality of ejaculate is going down, which is true for both human as well as domestic animals.[1,2] With the introduction of artificial insemination in domestic animals, the number of females served increased manifold; however, the conception rate remains low to date. Both males and females are responsible for the reduced fertility. Still, males are assumed to have a significantly higher influence on fertility prediction as ejaculates from bulls are used to cover thousands of females. Thus, it is a matter of great concern to identify underlying causes of male factor infertility/subfertility and to strengthen the selection criteria for breeding bulls. It has been found that cryopreserved spermatozoa are not superior in terms of achieving pregnancy in females as compared to liquid or fresh ejaculate. The growing demand to accelerate frozen semen production to cover at least 50% of breedable female bovines would include semen collection and processing from bulls with better cryotolerance.
Microencapsulation is a process by which cells or active ingredients are coated with polymeric materials as small droplets to maintain the functionality and longevity of coated cells and to increase shelf life.[3] Microencapsulation comprises bioencapsulation, indicating the entrapment of a biologically active substance.[4] Thus, bioencapsulation physically isolates a cell mass from an exterior environment and maintains normal cellular structure physiology within a semipermeable barrier.[5,6]
Following artificial insemination, millions of spermatozoa are deposited in the female reproductive tract. However, only a few hundred to thousand spermatozoa reach the site of fertilization, establish a reservoir, and wait for oocyte descendence. Thus, chances of fertilization and pregnancy establishment go down. The loss of sperm from the female reproductive tract is primarily by physical barriers such as cervical mucous as well as by immune attack (phagocytosis) by polymorphonuclear leukocytes.[7]
One ideal situation would be controlled utero release of packaged sperms [Figure 1], limiting retrograde transport and phagocytosis, preventing premature capacitation, and thus extending the fertilization competence of the inseminated sperm. This could alleviate the need for precise estrus determination and can save insemination dose. To maximize chances of fertilization, availability of higher numbers of spermatozoa at the site of fertilization and thus retention/prolonged release of spermatozoa would be beneficial in improving the conception rate. Therefore, microencapsulated spermatozoa could improve the success of artificial insemination.
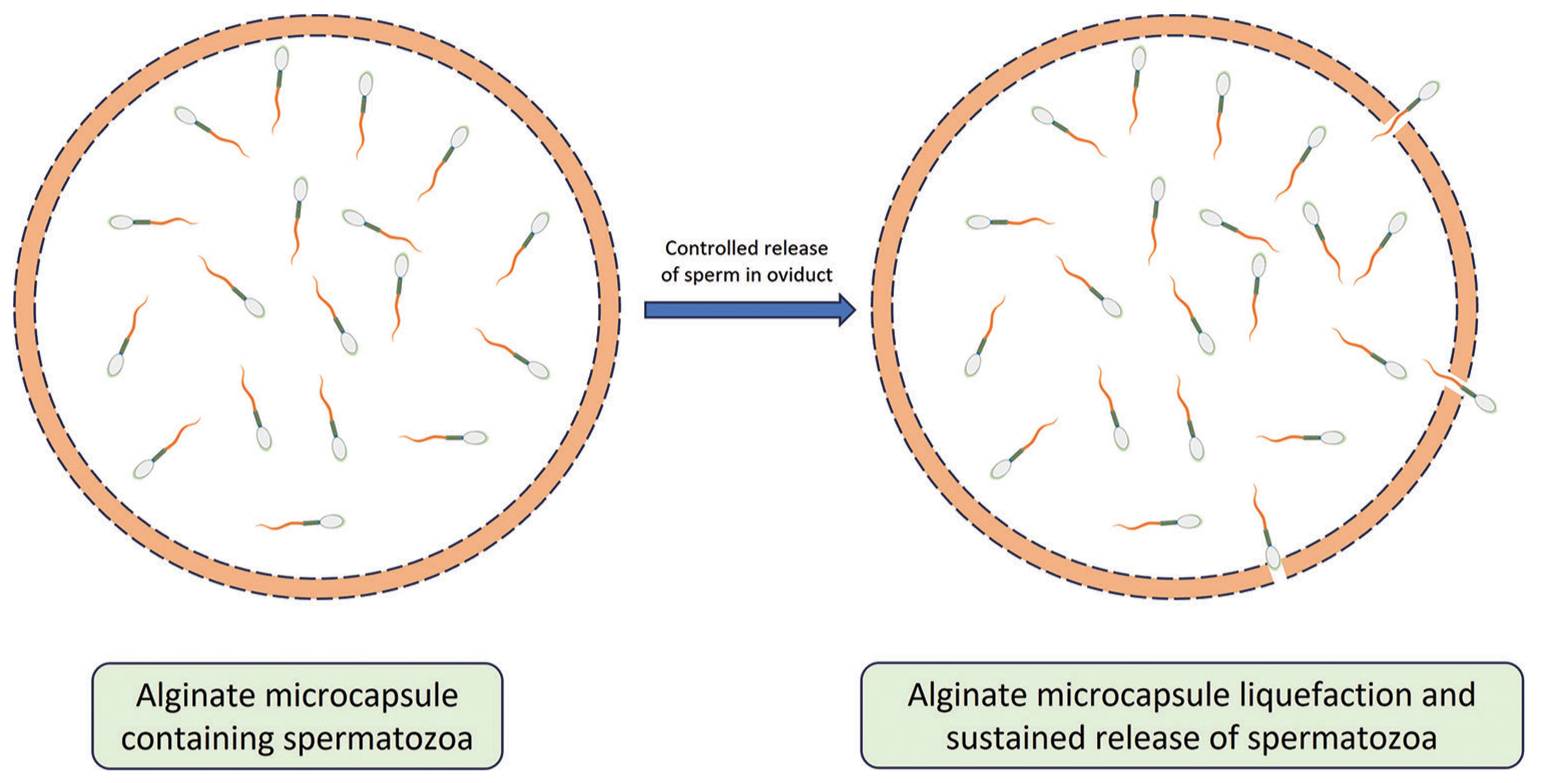
- Microencapsulated spermatozoa release in a controlled manner after liquefaction of microcapsule inside female oviduct.
HISTORY OF CELL MICROENCAPSULATION
One of the earliest reports on cell encapsulation was demonstrated back in 1933 when a tumor cell was polymer encapsulated and transplanted into a pig abdominal cavity, where cells survived a prolonged period by escaping host immune attack.[8] Three decades later, Chang developed microcapsules with semi-permeable membranes and called them “artificial cells,” which did not rely on membrane rupture for the transfer of encapsulated material.[9] In human regenerative medicine, microencapsulation has a potential role in in vivo cell delivery for repairing damaged tissue or organs. Two of the most practical applications for the microencapsulation of mammalian cells in human medicine include the microencapsulation of pancreatic cells in diabetes and hepatic cells for liver disease.[5]
TYPES OF MICROENCAPSULATING MATERIAL
Encapsulating materials can either be natural (polysaccharides, polypeptides, and polynucleotides) or synthetic. Among natural polymers, polysaccharides are mostly used due to their relatively smooth encapsulation processes that are compatible with cell viability (i.e., alginate, agarose, collagen, or cellulose). On the other hand, polyethylene glycol is considered the most widely used option among synthetic polymers, along with poly (lacticco-glycolic acid) and polyvinyl alcohol.[10] However, among all the available cell-encapsulating materials, alginate is considered to be the most widely used biomaterial due to its biocompatibility and easier handling.[11] Some of the popular methods and materials applied to cell encapsulation are shown in Table 1.
| Methods | Materials | References |
|---|---|---|
| Bulk hydrogel |
|
[15] [16] [17] [18] [19] [20] [21] |
| Hollow fibers |
|
[22] [23] [24] |
| Beads |
|
[25] [26] [27] [28] |
PEG: Polyethylene glycol, PLL: Poly-L-lysine, PAN-PVC: Poly (acrylonitrile-vinyl chloride), ADA: Alginate dialdehyde
Alginate is a natural anionic polysaccharide that creates three-dimensional structures (sol to gel), with increased divalent ions concentration. It consists of residues of β-Dmannuronic acid (M) and α-L-guluronic acid (G). The ratio of G and M influences some of the alginate hydrogel properties, such as biocompatibility, stability, mechanical resistance, and permeability. As a thumb rule, alginates with a higher proportion of G blocks are stiffer, compared to those with a higher proportion of M blocks that have better elastic properties due to the greater affinity of guluronic acid for divalent ions, and these compositional and physical-mechanical differences affect the way the immune system reacts against the implant. Studies showed that alginates having higher G content tend to show better compatibility and are thus best suited for cell encapsulation applications. [12] Crosslinking of anionic alginates with cationic compounds (poly-L-lysine/PLL) results in the production of microcapsules with controlled pore size.[13] However, utmost care is to be taken while crosslinking using PLL, as any unbound fraction of it can initiate an inflammatory response (PLL-induced tumor necrosis factor-alpha [TNFα] production in monocyte culture), as shown in the study.[14]
PURITY
The biocompatibility of encapsulated material, that is, alginate, is dependent on the degree of purity of the material. Low-purity alginates have been shown to contain lipopolysaccharides (endotoxin), peptidoglycans, and lipoteichoic acid, which reduce the biocompatibility of the implants and can damage the encapsulated cells.[29] Commercial alginates have been analyzed, and traces of pathogen-associated molecular patterns (PAMPs) were identified which are a potential trigger for the initiation of inflammatory processes. Endotoxins can bind to host toll-like receptors and result in the release of small proinflammatory cytokines (i.e., interleukin [IL] 1β, the TNF-α, or IL-6), which can interact with the encapsulated cells and cause damage.[30] After a lot of modification and purification, ultra-purified alginate microcapsules have been developed with effective removal of PAMPs.[31,32] The composition of purified alginate is described in Table 2.[32]
| Alginates | Ultra purification |
|---|---|
| Endotoxin | <1 EU/mL |
| Pyrogens | Absent |
| Proteins | <0.4% |
| Arsenic | 0.04 |
| Strontium | Undetectable |
| Iron | 0.65 |
| Manganese | 0.06 |
| Mercury | 0.02 |
| Magnesium | 0.72 |
| Lead | 0.03 |
| Copper | 0.15 |
| Zinc | Undetectable |
CHARACTERIZATION OF MICROCAPSULES
The formed microbeads/microcapsules can be characterized by their size, morphology, coating, water content, and thermal stability.
Size
A digital camera can carry out direct visualization of microcapsules with a macro lens. It can also be evaluated under a microscope and captured within a built camera. This provides a robust and rapid size characterization.
Micromorphological analysis
Clean grease-free microscopic slides are used to smear microbeads and to stain with 1% aqueous toluidine blue. The excess stain is then rinsed with distilled water, dried, and visualized under a microscope in oil immersion at higher magnification (×1000), which allows visualization of the integrity of cells entrapped within the capsule.
Scanning electron microscopy (SEM)
SEM is usually used at an accelerating voltage potential of 20 KV to investigate the topographical properties of microcapsules. Before examination, beads were prepared on aluminum stubs and coated with gold under an inert atmosphere.
Moisture content
It can be defined as the % weight of water with the dry weight and is a crucial factor in evaluating the viability of encapsulated cells. Microparticles of polysaccharide origin form gels have the capacity to retain water. Determined according to the methodology described in agreement with the Association of Official Analytical Chemists.[33]
METHOD OF SPERM ENCAPSULATION
For human ejaculation, semen samples are processed by density gradient centrifugation for sperm selection. The resultant sperm pellet is then diluted using sperm wash media. Before microencapsulation of spermatozoa, pure alginate solution in sperm wash media is made and vortexed for 30 min. The diluted sperm suspension is then mixed with alginate solution and extruded using a 0.8 mm diameter needle connected to a syringe in a cationic environment (CaCl2), which results in the hardening of microcapsule wall and formation of gel-like microbeads. The formed sperm containing microcapsules are then separated using a fine sieve and washed by sperm wash media to remove excess CaCl2. Confirmation of microcapsules can be done by visualization using the phase-contrast microscope and Diff-Quick stain, whereas vital sperm functional tests can be done using fluorescent dyes in a time-dependent manner aided by a fluorescent microscope or flow cytometer.
The modification of this sperm microencapsulation protocol could be used in other species, including bulls, as mentioned by many authors.[7,34,35] Briefly, extended bull semen is added to sodium alginate solution (1% w/v). The alginate–sperm suspension is then added drop-wise with a needle (32 G) into a continuously stirred semen extender containing divalent cations (CaCl2/BaCl2). The divalent ions react with the alginate chains and form alginate gel beads, which are then separated after washing and filtered through a fine sieve as described previously. The encapsulated spermatozoa are then evaluated for vital parameters and resuspended in an extender, loaded in straws, and cryopreserved using standard protocols.[36] The potential of sperm microencapsulation for enhancing conception rates in bovines is presented in Figure 2.
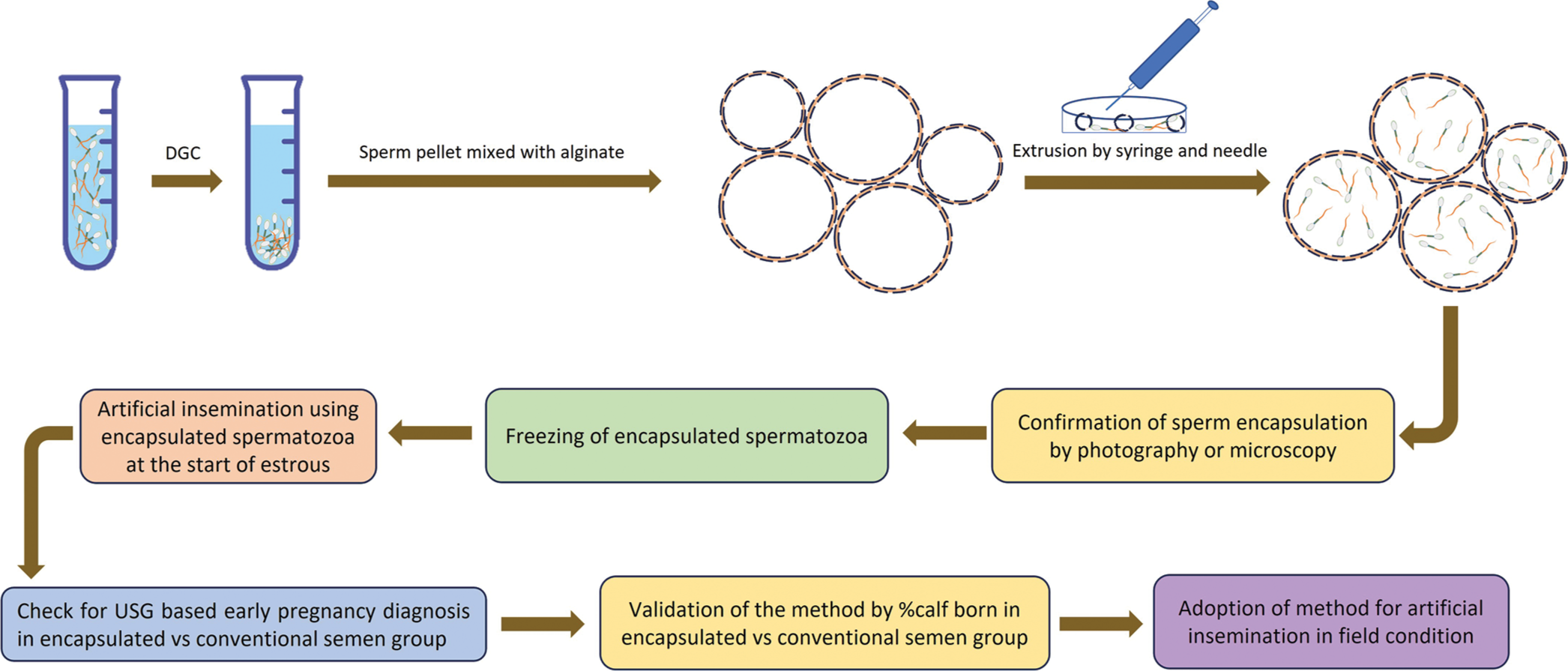
- Schematic representation of the use of microencapsulated spermatozoa in enhancing conception rate in bovines. Semen collected by artificial vagina method is first centrifuged to obtain sperm pellet. The resultant sperm pellet is then mixed with alginate microcapsules in solution and extruded using a syringe and needle. Extrusion results in a temporary break in the capsule wall and entry of spermatozoa inside. The capsule wall break closes following extrusion and the presence of spermatozoa inside the microcapsule can be confirmed through bright-field as well as fluorescent microscopy. Microencapsulated spermatozoa are then used for insemination or frozen in liquid nitrogen for future use.
USE OF ALGINATE MICROCAPSULES IN BOVINE REPRODUCTION
The first report on bovine sperm microencapsulation was done back in 1985 by Nebel et al.,[7] where three different concentrations of spermatozoa were used, and the encapsulated sperm were analyzed for viability and motility in a time-dependent manner. They found that viability and acrosome integrity were maximal for encapsulated spermatozoa in 15% and 10% egg yolk-containing extenders. They suggested that microencapsulated bull spermatozoa could be used to improve the success of artificial intelligence (AI).
For controlled sperm release during artificial insemination, a study was designed with two strategies for the automated microencapsulation of bovine spermatozoa in either alginate– Ca2+ or cellulose sulfate (CS)-poly-diallyldimethyl ammonium chloride (pDADMAC) capsules. These capsules were easy to produce, enabled encapsulation of high motile sperms, were well-suited with standard freezing protocols, and could be degraded for controlled sperm release after the addition of pure cellulase or cellulase entrapped in alginate hydrogels. In vitro, data indicated that CS-pDADMAC-based sperm encapsulation offered high standards of sperm protection.[37]
In another study, encapsulated spermatozoa were cryopreserved for the 1st time in buffalo, and insemination was carried out using frozen-thawed encapsulated spermatozoa. In vitro, semen analyses revealed that the encapsulation process did not deteriorate semen quality either before or after cryopreservation in buffaloes. In buffalo, the pregnancy rates were higher for encapsulated spermatozoa as compared to that in the control group (P > 0.05). Encapsulated spermatozoa were also used for inseminating synchronized heifers and pregnancy rates were comparable to the control group.[36]
CONCLUSION AND FUTURE ASPECTS
Two areas of bovine reproduction where microencapsulated semen may have a future advantage over conventional methods are with superovulated donor cows and in estrous synchronization programs. In these situations, ovulation occurs over an extended period of time; thus, controlled release from microcapsules could make sperm available over an extended period. Thus, microencapsulation offers the possibility of reducing the need for timing of AI and can be of great use where there is a shortage of veterinarians or AI technicians. However, a thorough characterization of the immune mechanisms involved in anti-capsular response is important for successful in vivo implementation of the technology.
Authors’ contributions
NP conceptualized the review. TRT was involved in manuscript formatting. AK did a manuscript review and approved the manuscript for publication.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient consent not required as there are no patients in this study
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Decreasing sperm quality: A global problem? BMC Public Health. 2010;10:1-5.
- [CrossRef] [PubMed] [Google Scholar]
- Breeding soundness evaluation in crossbred bulls: Can testicular measurements be used as a tool to predict ejaculate quality. Indian J Anim Sci. 2014;84:177-80.
- [CrossRef] [Google Scholar]
- Microencapsulation for the therapeutic delivery of drugs, live mammalian and bacterial cells, and other biopharmaceutics: Current status and future directions. J Pharm. 2013;2013:103527.
- [CrossRef] [PubMed] [Google Scholar]
- Microencapsulation of living cells and tissues. J Pharm Sci. 1981;70:351-4.
- [CrossRef] [PubMed] [Google Scholar]
- Technology of mammalian cell encapsulation. Adv Drug Deliv Rev. 2000;42:29-64.
- [CrossRef] [PubMed] [Google Scholar]
- Microencapsulation of bovine spermatozoa. J Anim Sci. 1985;60:1631-9.
- [CrossRef] [PubMed] [Google Scholar]
- Uber die antineoplastische immunitat; heterologe Einpflnzung von Tumoren in Huhner-embryonen. Ztschr Krebsforsch. 1933;40:122-40.
- [CrossRef] [Google Scholar]
- Cell microencapsulation with synthetic polymers. J Biomed Mater Res Part A. 2015;103:846-59.
- [CrossRef] [PubMed] [Google Scholar]
- Alginate: Properties and biomedical applications. Prog Polym Sci. 2012;37:106-26.
- [CrossRef] [PubMed] [Google Scholar]
- Factors influencing the mechanical stability of alginate beads applicable for immunoisolation of mammalian cells. J Mech Behav Biomed Mater. 2014;37:196-208.
- [CrossRef] [PubMed] [Google Scholar]
- Polymeric materials for perm-selective coating of alginate microbeads. Methods Mol Biol. 2017;1479:95-109.
- [CrossRef] [PubMed] [Google Scholar]
- Poly-L-lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant. 2001;10:263-75.
- [CrossRef] [Google Scholar]
- PEG-based hydrogels with collagen mimetic peptide-mediated and tunable physical cross-links. Biomacromolecules. 2010;11:2336-44.
- [CrossRef] [PubMed] [Google Scholar]
- Silk fibroin/poly (vinyl alcohol) photocrosslinked hydrogels for delivery of macromolecular drugs. Acta Biomater. 2012;8:1720-9.
- [CrossRef] [PubMed] [Google Scholar]
- Photopolymerization of cell-encapsulating hydrogels: Crosslinking efficiency versus cytotoxicity. Acta Biomater. 2012;8:1838-48.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous chondrocyte maturation on 3d-chitosan scaffolds. J Tissue Sci Eng. 2013;4:1.
- [CrossRef] [Google Scholar]
- Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013;9:7081-92.
- [CrossRef] [PubMed] [Google Scholar]
- Structure and function of laminin: Anatomy of a multidomain glycoprotein. FASEB J. 1990;4:148-60.
- [CrossRef] [PubMed] [Google Scholar]
- Review of the characterization of sodium alginate by intrinsic viscosity measurements: Comparative analysis between conventional and single point methods. Int J Biomater Sci Eng. 2014;1:1-11.
- [Google Scholar]
- Antiangiogenic gene therapy in inhibition of metastasis. Acta Biochim Pol. 2002;49:313-21.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of fabrication conditions on the structure and function of membranes formed from poly (acrylonitrile-vinylchloride) J Membrane Sci. 1998;147:235-45.
- [CrossRef] [Google Scholar]
- Evaluation of adhesion, proliferation, and functional differentiation of dermal fibroblasts on glycosaminoglycan-coated polysulfone membranes. Tissue Eng Part A. 2008;14:1687-97.
- [CrossRef] [PubMed] [Google Scholar]
- Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014;10:3650-63.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of viability and proliferation of alginate-poly-L-lysine-alginate encapsulated myoblasts using flow cytometry. J Biomed Mater Res Part B Appl Biomater. 2010;94:296-304.
- [CrossRef] [PubMed] [Google Scholar]
- Alginate and chitosan polyion complex hybrid fibers for scaffolds in ligament and tendon tissue engineering. J Orthop Sci. 2005;10:302-7.
- [CrossRef] [PubMed] [Google Scholar]
- Designing biopolymer fluid gels: A microstructural approach. Food Hydrocoll. 2014;42:362-72.
- [CrossRef] [Google Scholar]
- Impact of residual contamination on the biofunctional properties of purified alginates used for cell encapsulation. Biomaterials. 2006;27:1296-305.
- [CrossRef] [PubMed] [Google Scholar]
- Immunological challenges facing translation of alginate encapsulated porcine islet xenotransplantation to human clinical trials. Methods Mol Biol. 2017;1479:305-33.
- [CrossRef] [PubMed] [Google Scholar]
- A technology platform to test the efficacy of purification of alginate. Materials. 2014;7:2087-103.
- [CrossRef] [PubMed] [Google Scholar]
- Microencapsulation for cell therapy of type 1 diabetes mellitus: The interplay between common beliefs, prejudices and real progress. J Diabetes Invest. 2018;9:231.
- [CrossRef] [PubMed] [Google Scholar]
- Sperm encapsulation from 1985 to date: Technology evolution and new challenges in swine reproduction. Reprod Domest Anim. 2015;50:98-102.
- [CrossRef] [PubMed] [Google Scholar]
- Boar sperm changes after sorting and encapsulation in barium alginate membranes. Theriogenology. 2013;80:526-32.
- [CrossRef] [PubMed] [Google Scholar]
- Alginate encapsulation preserves the quality and fertilizing ability of Mediterranean Italian water buffalo (Bubalus bubalis) and Holstein Friesian (Bos taurus) spermatozoa after cryopreservation. J Vet Sci. 2017;18:81-8.
- [CrossRef] [PubMed] [Google Scholar]
- Design of high-throughput-compatible protocols for microencapsulation, cryopreservation and release of bovine spermatozoa. J Biotechnol. 2006;123:155-63.
- [CrossRef] [PubMed] [Google Scholar]






