Translate this page into:
Advancements in siRNA delivery using nanotechnology for reproductive tract cancers: From targeting to enhancing treatment efficacy

*Corresponding author: Dinesh Kumar Mishra, Department of Pharmacy, Guru Ghasidas Vishwavidyalaya (A Central University), Bilaspur, Chhattisgarh, India. dineshdops@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Jangde KK, Mishra DK. Advancements in siRNA delivery using nanotechnology for reproductive tract cancers: From targeting to enhancing treatment efficacy. J Reprod Healthc Med. 2024;5:6. doi: 10.25259/JRHM_13_2024
Abstract
Cancers of the reproductive system, which include those of the cervix, uterus, ovaries, fallopian tubes, and vagina, are major global health concerns. Even with improvements in traditional therapies such as radiation and chemotherapy, managing these tumors is still difficult, frequently associated with side effects, and has limited effectiveness. Targeted cancer therapy now has better options, thanks to the development of small interfering ribonucleic acid (siRNA) as a potentially effective therapeutic procedure through the ribonucleic acid interference (RNAi) technique in recent years. Nevertheless, there are many challenges in the clinical translation of siRNA, especially in terms of delivering drugs effectively to tumor-specific areas while reducing off-target effects. Nanotechnology has emerged as a transformative perspective that could show a promising approach for providing a revolutionary solution to the problems related to siRNA delivery. By carefully crafting nanocarriers, including liposomes, polymeric nanoparticles, and lipid nanoparticles, scientists have made incredible strides toward improving the bioavailability, stability, efficacy, and selectivity of siRNA treatments. Furthermore, due to their customizable characteristics, nanocarriers could be used to target specific tumors of the reproductive tract, enhancing the effectiveness of the treatment by taking advantage of tumor-specific indicators and microenvironments. In this review, we will summarize and delve into leveraging the synergistic benefits of targeted delivery and enhanced therapeutic potency using the RNAi technique. These innovative strategies hold immense potential to reshape the treatment landscape, offering new hope for patients with these devastating reproductive tract malignancies.
Keywords
Reproductive tract cancers
Ovarian cancer
siRNA
Ribonucleic acid interference
Nanocarriers
INTRODUCTION
Gynecological malignancies are the most prevalent cancer among women, and they represent a significant public health concern. A large percentage of women report the disease at advanced stages, which has a negative impact on the prognosis and clinical results. The anticipated number of new gynecological cancer cases and fatalities in 2003 was 83,700 and 26,800, respectively. The American Cancer Society reported in 2018 that the prevalence of gynecological cancer was approximately 89,000, with 29,000 casualties. Moreover, 13,240 cases of cervical cancer were reported in the United States in 2019, among whom 4170 people lost their lives. The American Cancer Society also reported ovarian cancer, with 22,240 patients and 14,070 deaths in 2018.[1] According to the Indian Council of Medical Research’s National Cancer Registry Program (NCRP), more than 3.4 lakh active patients with cervical cancer were reported in India in 2023.
An estimated 5 lakh women worldwide are diagnosed with gynecological cancer each year, and over 1 lakh new patients receive a diagnosis in India alone, as reported in 2018 by the American Oncology Department. This is because impoverished countries like India lack adequate screening facilities, have complex pathologies, and lack awareness of cancer. In India, ovarian cancer has become the most prevalent cancer to affect women, followed by cervical cancer, and its incidence rates have been rising over time.[2]
In this article, we focused on presenting personalized therapy for reproductive tract cancer through targeted medicines and accompanying biomarkers that may be delivered using nanocarrier materials for siRNA distribution and co-delivery with anticancer drugs. The focus is on the synthesis, design, and characterization of the aforementioned supplies through adopting the efficient and reliable nanotechnology-based approach.
DEMOGRAPHIC PREVALENCE OF REPRODUCTIVE TRACT CANCERS
The coronavirus disease of 2019 (COVID-19) pandemic in 2020 resulted in postponements in cancer detection and treatment due to healthcare facility closures, changes in work and health insurance policies, and worry about being exposed to COVID-19. There has been a recent prediction by the National Institutes of Health (NIH) suggesting that by 2026, there will be 0.934 million new cases altogether among males, an increase from 0.589 million in 2011. In a similar fashion, the number of new cancer cases in females, as predicted by the NIH, rose from 0.603 million in 2011– 0.935 million by 2026. In addition, among young people, the incidence rates of colorectal cancer (age <55) and cervical cancer (age 30–44) have increased by 1–2% yearly since 2004. According to the NCRP, the most prevalent malignancies in women were cervix uteri and breast cancer. In India, 6–29% of all malignancies in women were cervical cancers, as per the data published by the NCRP in March 2016. As per the data for 2020 from the Global Cancer Observatory (GLOBOCAN), with an incidence rate of 18.3% (123,907 cases), cervix uteri cancer is the third most common type of cancer overall and the second most common cause of death in India, accounting for 9.1% of all deaths.[3,4]
VITALITIES ASSOCIATED WITH REPRODUCTIVE TRACT CANCERS
Reproductive tract cancers encompass a range of malignancies that affect the female reproductive system. The incidence of reproductive tract malignancies varies by geography and is also influenced by socioeconomic variables, uncontrolled hormonal contraceptive use, cultural norms, and healthcare facilities. Incidence rates and mortality rates for female reproductive tract cancers vary widely by geographic region, with higher rates being witnessed in poor and middle-income nations due to restricted availability of screening, safeguarding, and treatment initiatives. Socioeconomic factors, cultural practices, and health-care infrastructure also influence the burden of reproductive tract cancers in different regions.[5,6]
In preclinical models, many cancer treatment regimens have been implemented. For decades, traditional chemotherapy with cytotoxic drugs was the primary treatment for reproductive tract cancer. The complicated genomics and molecular anomalies in reproductive tract cancer present challenges for precise risk classification and targeted therapy, yet there are opportunities for the improvement of the approach toward cancer therapy. New molecular tools, such as next-generation sequencing and ribonucleic acid interference (RNAi) techniques, have cleared the way for new medicine development that targets specific gene variants.[7] New therapies for reproductive tract cancer include T-cell engager antibodies, antibody-drug conjugates, immune checkpoint inhibitors, metabolic and pro-apoptotic drugs, and modified T-cells have all been developed.[8,9] Personalized therapies possess the capacity to deliver highly proficient anti-cancer treatment with scarcely any adverse consequences compared to normal cytotoxic chemotherapies for the treatment of reproductive tract cancer. Patients may be chosen for therapy depending on either the availability or lack of certain molecular targets, ensuring the highest likelihood of response while minimizing unnecessary toxicity. Targeting the entire tumor is challenging as different subclones of reproductive tract cancer cells have distinct biological characteristics.[10]
OVERVIEW OF COMMON REPRODUCTIVE TRACT CANCERS (e.g., OVARIAN, CERVICAL, AND ENDOMETRIAL CANCERS)
The female vaginal tract is the most prevalent location for tumors in females. The uterine corpus is the second most prevalent location for female genital malignancies. The main types of female reproductive tract cancer include ovarian cancer, cervical cancer, uterine (endometrial) cancer, vaginal cancer, and vulvar cancer.[11] These cancers originate from various tissues within the reproductive organs, which include the ovary, cervix, uterine (endometrial) wall, vagina, and vulva. Each type of cancer associated with the female reproductive tract has its own distinct characteristics, risk factors, and treatment approaches. A brief description of female reproductive tract cancer has been briefly discussed below: [12,13]
Ovarian cancer
Ovarian cancer originates in the ovaries, which are in the position of generating female hormones and eggs. Because symptoms may not appear until the disease attains an advanced stage; therefore, it is commonly referred to as the “silent killer.” Age, family history, inherited genetic mutations such as BReast CAncer gene 1 and 2 (BRCA1 and BRCA2), and hormone therapy are among the probable risk factors. Ovarian cancer is characterized by complex molecular alterations that contribute to its development, progression, and therapeutic response. Mutations in the tumor protein 53 (TP53) tumor suppressor gene are prevalent in high-grade serous ovarian cancer. TP53 mutations lead to impaired deoxyribonucleic acid (DNA) damage, leading to genomic instability. Germline mutations in BRCA1 and BRCA2 genes predispose individuals to hereditary ovarian cancer. These mutations impair DNA repair mechanisms. Additional mutations in genes such as phosphatase and tensin homolog (PTEN), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit-alpha (PIK3CA), and Kirsten rat sarcoma viral oncogene homolog (KRAS) can drive ovarian cancer progression by dysregulating cell cycle control, apoptosis, and signaling pathways. The epigenetic alterations such as DNA methylation and dysregulation of histone acetylation, methylation, and phosphorylation can alter chromatin structure, affecting gene expression and cancer progression.[14,15] Treatment typically involves surgery, chemotherapy, and sometimes targeted therapies. Molecular profiling of ovarian cancer tumors helps in stratifying patients for personalized therapies. One example is poly (ADP-ribose) polymerase (PARP) inhibitors for BRCA-mutated cancers. Molecular mechanisms underlying resistance to chemotherapy (e.g., platinum resistance) and targeted therapies (e.g., PARP inhibitors) pose challenges in effective treatment of ovarian cancer. Understanding these molecular details is crucial for developing targeted therapies and improving clinical outcomes for patients with ovarian cancer.[16,17]
Cervical cancer
Cervical cancer could be developed by cells found in the cervix, the area of the uterus below the vagina. Human papillomavirus (HPV) infections that are high-risk, specifically HPV-16 and HPV-18, are the main cause of cervical cancer. The E6 and E7 oncoproteins of HPV play crucial roles in this process by inhibiting tumor suppressor proteins TP53 and retinoblastoma protein, respectively, leading to uncontrolled cell proliferation and genomic instability.[18,19] Precancerous alterations can be identified early with routine Pap and HPV testing screening, enabling management before cancer emerges. Surgery, radiation therapy, chemotherapy, or a mix of these techniques could be used as a form of treatment.[20,21]
Uterine (endometrial) cancer
Uterine cancer originates in the endometrium that constitutes the uterine lining. The development of this cancer often involves estrogen-driven proliferation of endometrial cells, coupled with genetic alterations such as mutations in PTEN, PIK3CA, and CTNNB1 genes, which disrupt normal cellular signaling pathways regulating growth and apoptosis. Endometrial cancer is also associated with obesity and metabolic syndrome, which contribute to increased estrogen levels and chronic inflammation, further promoting tumorigenesis.[22] Changes in bowel or urine habits, pelvic pain, and irregular vaginal bleeding are prospective signs. Understanding these molecular mechanisms is crucial for developing targeted therapies. Treatment typically involves surgical intervention to remove the uterus (hysterectomy) and may be followed by radiation therapy or chemotherapy.[23,24]
Vaginal and vulvar cancers
Vaginal cancer originates in the cells lining the vagina, while vulvar cancer arises in the external genitalia (vulva). These cancers are relatively rare compared to other reproductive tract malignancies. Vaginal cancer often arises from precursor lesions, most commonly squamous cell carcinoma, adenocarcinoma, or melanoma. The majority of vaginal cancers are associated with HPV infection, particularly high-risk types such as HPV-16 and HPV-18.[25] Other risk factors may include aging, smoking, and a history of precancerous lesions. Strategies for treatment include surgery, radiation therapy, chemotherapy, and targeted therapy, depending on the stage of cancer and its location.[26-28]
In a nutshell, it may be suggested that early detection and advances in treatment have improved outcomes for many patients with reproductive tract cancers. However, ongoing research is focused on developing more effective screening methods, targeted therapies, and personalized treatment approaches to improve further survival rates and quality of life for individuals affected by these cancers.
INVOLVEMENT OF siRNA: A TARGET-BASED GENE THERAPY
Targeted messenger ribonucleic acids (mRNAs) are subjected to sequence-specific destruction, in response to double-stranded ribonucleic acid (dsRNA) when performed on Caenorhabditis elegans, a nematode. This kind of procedure is termed as RNAi. RNAi is a novel process of molecular genetics that causes sequence-specific gene suppression by implementing RNA molecules, more specifically dsRNA, through adapting the basic genetic process, that is, translational or transcriptional repression.[29] Post-transcriptional gene silencing, a related process that was first identified in plants years ago, is currently being considered as a surveillance mechanism to prevent the synthesis or expression of potentially hazardous RNAs.[30-32] The use of long dsRNA (>38 base pairs) for RNAi detection in mammalian cells is impeded by the activation of the interferon response pathway, which prevents the production of protein throughout. Elbashir et al. revealed that certain genes are targeted and could be inhibited by siRNAs, which are 21 nucleotides produced from lengthy dsRNA during RNAi, making it easier to prevent RNA expression in somatic mammalian cells which was also reported earlier. After these historic accomplishments, plenty of in vitro and in vivo studies have been conducted, with assurance for therapeutic utilization and its impact on medical science.[33,34]
The molecular aspects of siRNA in reproductive cancers involve gene targeting, an effective delivery system, and specific gene silencing. In particular, the gene targeting in reproductive cancers such as ovarian, cervical, or endometrial cancers exhibits specific genetic alterations that drive their pathogenesis. siRNA can target key genes involved in cell proliferation (e.g., Cyclin D1), apoptosis (e.g., Bcl-2), angiogenesis (e.g., vascular endothelial growth factor), and drug resistance (e.g., ATP-binding cassette or ABC transporters).[35] The delivery system for the siRNA is a very crucial part which requires various methods that include lipid-based nanoparticles, polymer-based carriers, or viral vectors in terms of cellular uptake and specificity to the target.[36]
Apart from that, the genetic aspects of siRNA in reproductive tract malignancy include gene expression profiling and resistance mechanisms. Knowing the genomic landscape aids in the selection of siRNA targets that will effectively disrupt particular pathways responsible for the progression of cancer. Studies on transcriptomes have shown dysregulated genes and signaling networks in malignancies of the reproductive system, offering prospective targets for siRNA-based treatments.[37]
The resistance mechanism assisting the therapy of reproductive tract cancer is attributed to resistance to traditional therapy in malignancies of the reproductive system and is influenced by genetic mutations and alterations. Furthermore, by avoiding specific obstacles, siRNA targeting of genes could be engaged in drug resistance pathways (such as epidermal growth factor receptor mutations in ovarian cancer) and that may overcome resistance and improve treatment outcomes.[38]
siRNA DETERMINED THERAPEUTIC TARGETS IN OVARIAN CANCER: A TARGETED STRATEGIES
The DNA, RNA, and proteins are interchangeably used for gene therapy due to their active gene regulation and modulation properties. The basic messenger of genetic material is RNA, which actively takes part in the transmission of genetic information. RNA can precisely carry out the gene expression, formation of proteins, downstream cellular pathways, sequence-specific interactions, and so on. One way of gene regulation and modulation is the RNAi technique.[39] RNAi, being an endogenous process, works by the mechanism of reduction of protein expression through hindering the translation process of mRNA. MicroRNAs and siRNAs are the biological mechanisms by which RNAi processes are triggered. siRNA has emerged as a prospective therapeutic alternative for a variety of ailments, including cancer, genetic disorders, viral infections, and neurological diseases. This ground-breaking method selectively silences target genes through the utilization of the natural RNAi pathway, providing a very potent and specific tool for amending gene expression. The mode of action of siRNA is attained when it operates through the RNAi pathway, a highly ubiquitous cellular mechanism that controls post-transcriptional gene expression. The first step in the process is the formation of double-stranded siRNA molecules that are complementary to the target mRNA sequence. These molecules are usually 21–23 nucleotides long. After infiltrating the cell, siRNA molecules engage with the RNA-induced silencing complex (RISC), where they utilize base-pairing interactions to guide the RISC toward the target mRNA. The target gene’s expression is effectively silenced when the RISC cleaves the target mRNA, leading to its destruction and consequent suppression of protein synthesis.[40]
In the study conducted in 2011, by Cheung et al., they discovered 54 oncogenes that are responsible for the survival and growth of ovarian cancer. Some of the types of oncogenes involved in ovarian cancer include BRCA1, BRCA2, BRIP1, MLH1, MSH2, MSH6, RAD51C, and RAD51D. The strategies adopted for the mitigation of ovarian cancer using siRNA-conjugated nanosystems include knocking out the oncogenes that support oncogene proliferation. Then, the gene down regulation mechanism is employed to reduce the metastasis and induce/restore the programmed cell death process. Furthermore, it is followed by suppression or downregulation of ovarian cancerous cells responsible for drug resistance.[41]
THE PROSPECT OF siRNA-LOADED DRUG DELIVERY TO THE TARGETED CELLS
In therapeutic medicine, siRNA-loaded medication delivery to specific cells is a novel method, especially in genetic disorders and oncology. With the use of small interfering RNA (siRNA) molecules, disease-related genes can be precisely silenced at the mRNA level, allowing for precise control over cellular activities that are essential for the progression of the disease. The purpose of this focused strategy is to reduce systemic adverse effects that are frequently linked to traditional medicines while simultaneously improving treatment efficacy.[42] Despite their potential for treating reproductive tract cancer, siRNA medicines face numerous biological hurdles following systemic delivery before reaching the target mRNA. Roche Pharmaceuticals and other large pharmaceutical corporations retracted their investment in siRNA treatments due to the lack of effective ways to address technical obstacles such as siRNA-based drug delivery. These difficulties arise during blood circulation, overcoming physiological barriers, gaining access to specific areas of tissues and cells, cellular endocytosis, and intracellular mobility. Fortunately, recent advances in nanotechnology have resulted in unprecedented opportunities and tools for resolving these biological obstacles, making siRNA increasingly efficient in the treatment of reproductive tract cancer. The most common method for delivering drugs loaded with siRNA is to encapsulate siRNA molecules in nanoparticles or other carriers. These delivery vehicles help target cells absorb siRNA by preventing it from degrading. Once within the cells, siRNA directs the RISC to mRNA sequences that are complementary, which causes the degradation of those sequences and the consequent suppression of protein expression.[43] Precision for particular genes or pathways linked to diseases like cancer increased potency through disease progression suppression by blocking important molecular targets, and versatility in a broad range of diseases beyond cancer, such as genetic disorders and viral infections, are some of the benefits of siRNA-loaded drug delivery. This article discussed the problems and ways for overcoming the above by utilizing a nanotechnology approach using state-of-the-art nano-carrier substituents, such as nucleic acid nano-carriers, solid lipid nanoparticles, biodegradable polymer nanoparticles, liposomes, dendrimers, prodrugs, and micelles.[44,45]
Targeted therapy plays a pivotal role in improving the therapy associated with those with tumors of the reproductive tract, including the endometrial, cervical, and ovarian cancers. Future advancements in siRNA-loaded drug delivery aim to expand the repertoire of targetable genes and diseases, enhance delivery efficiency and reduce toxicity, and incorporate combination therapies to synergistically enhance treatment outcomes.[46] Here are some key points depicting the reasons why targeted therapy is important in the management of these cancers: [47,48]
Precision medicine
Targeted therapy allows for a more precise and individualized approach to treatment. By specifically targeting these molecular abnormalities or signaling pathways, it could drive cancer growth as well as progression. Targeted therapies have the ability to adapt to the particular environment of each patient’s tumor. This personalized strategy would reduce the needless toxicity to the healthy tissues while optimizing therapeutic efficacy.
Enhanced therapeutic efficacy
Targeted therapies offer the potential for improved therapeutic outcomes in contrast to traditional therapies such as radiation or chemotherapy. By directly blocking particular molecular targets that are significant in the survival, proliferation, and metastasis of cancer cells, targeted therapies may exert potent antitumor effects with fewer off-target effects. This could lead to expanded cumulative patient survival, extended progression-free survival times, and faster response rates with reproductive tract cancers.
Overcoming resistance
Reproductive tract cancers can eventually grow resistant to traditional therapies, which could result in inadequate or failed treatment and amplification of the illness. Targeted therapies may assist in overcoming resistance mechanisms by concentrating on different signaling pathways or weaknesses present in the tumor cells. Combinatorial approaches combining targeted therapies with other treatment modalities may synergistically target multiple pathways and circumvent resistance, improving treatment outcomes for patients.
Reduced toxicity
Unlike conventional treatments, which often cause systemic toxicity due to their non-specific nature, targeted therapies selectively target cancer cells while sparing normal tissues. This focused strategy reduces adverse effects of treatment and enhances patients’ quality of life both during and after treatment. By reducing treatment-related toxicity, targeted therapies may also allow for higher treatment doses or prolonged treatment durations, further enhancing therapeutic efficacy.
Management of advanced disease
Patients with advanced or recurrent reproductive tract malignancies may have few therapy options. In such a scenario, targeted medicines could provide a new ray of hope. In cases where surgery or chemotherapy is no longer effective, targeted therapies could provide meaningful clinical benefits, including disease stabilization, symptom relief, and improved quality of life.
Potential for combination therapies
To create synergistic effects and improve treatment outcomes, targeted therapies may be used in conjunction with other treatment methods such as immunotherapy, radiation therapy, or chemotherapy. Combinatorial approaches that target multiple pathways or overcome resistance mechanisms hold promise for improving response rates and prolonging survival in patients with reproductive tract cancers.
In summary, targeted therapy represents a valuable therapeutic approach in the management of reproductive tract cancers, offering precision, efficacy, and reduced toxicity compared to conventional treatments. As our understanding of the molecular drivers of these cancers continues to deepen, targeted therapies will play an increasingly important role in optimizing treatment strategies and improving outcomes for patients affected by reproductive tract cancers.[49]
siRNA DELIVERY USING NANO-FORMULATIONS AND TUMOR-SPECIFIC LIGANDS/MOLECULAR TARGETING
One of the key advantages of siRNA therapy is its exquisite specificity and potency in targeting individual genes. Highly selective gene silence may be achieved by creating siRNA molecules that are complementary to the mRNA sequence of a particular target gene without harming non-targeted genes. This specificity minimizes off-target effects and reduces the risk of unintended consequences, making siRNA an attractive therapeutic option for precision medicine applications.
siRNA therapy offers versatility in targeting a wide range of disease-causing genes, including those that are difficult to target using traditional small molecules, drugs, or antibodies. This includes targets such as oncogenes, viral genes, mutant alleles, and genes involved in pathogenic processes.[50] Furthermore, siRNA could be designed to target multiple genes simultaneously or specific splice variants or isoforms, providing flexibility in addressing complex disease mechanisms [Figure 1].
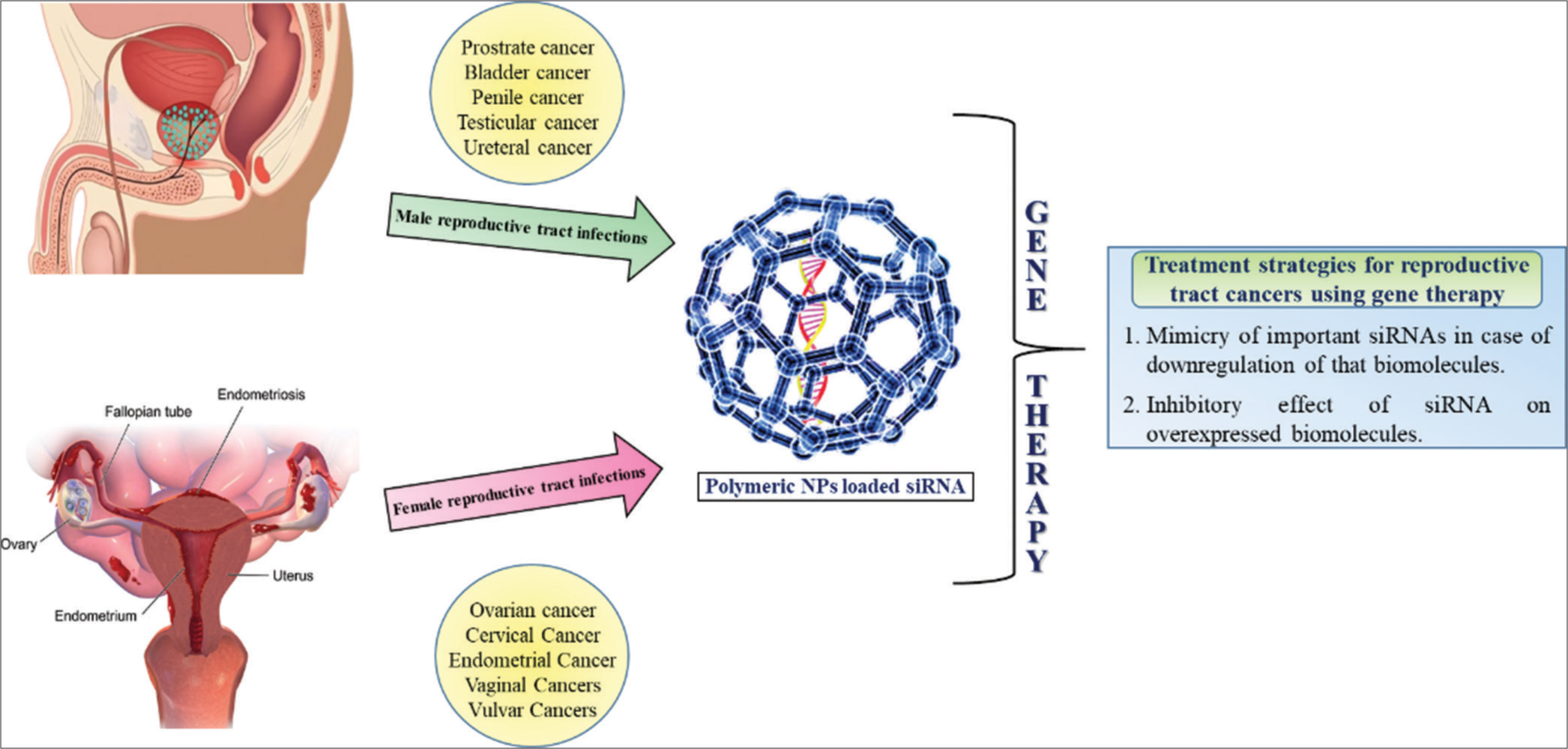
- Overview of the use of nanomaterials for the diagnosis and treatment of reproductive tract cancers.
Therefore, siRNA is a revolutionary method of governing genes that has great therapeutic promise for treating a variety of ailments. As our understanding of RNAi biology continues to deepen and technological innovations continue to evolve, siRNA-based therapeutics are poised to revolutionize medicine by offering precise and potent treatments tailored to individual patients’ needs.[51]
HINDRANCE IN siRNA DELIVERY FOR EFFECTIVE THERAPEUTIC OUTCOMES
The potential therapeutic applications of siRNA are vast and encompass various fields of medicine. Using siRNA to target oncogenes, tumor suppressor genes, or drug-resistance genes in oncology is a fresh strategy for cancer treatment. In infectious diseases, siRNA could inhibit the replication of viruses or other pathogens by targeting essential viral genes or host factors required for viral replication. In addition, siRNA holds promise for the prognosis of inherited genetic illnesses, neurodegenerative diseases, inflammatory conditions, and metabolic disorders.
Despite the tremendous potential of siRNA, the smooth delivery is still a concern. Among the difficulties facing siRNA therapy include immunological exhilaration, off-target repercussions, and transport hurdles. To overcome these obstacles, siRNA chemistry and design shall be optimized, as well as effective and secure delivery methods have to be developed, and careful selection of target genes is indeed needed [Figure 2]. However, ongoing advancements in nanotechnology, RNA chemistry, and delivery technologies are rapidly addressing these challenges, paving the way for the clinical translation of siRNA-based therapeutics.[52]
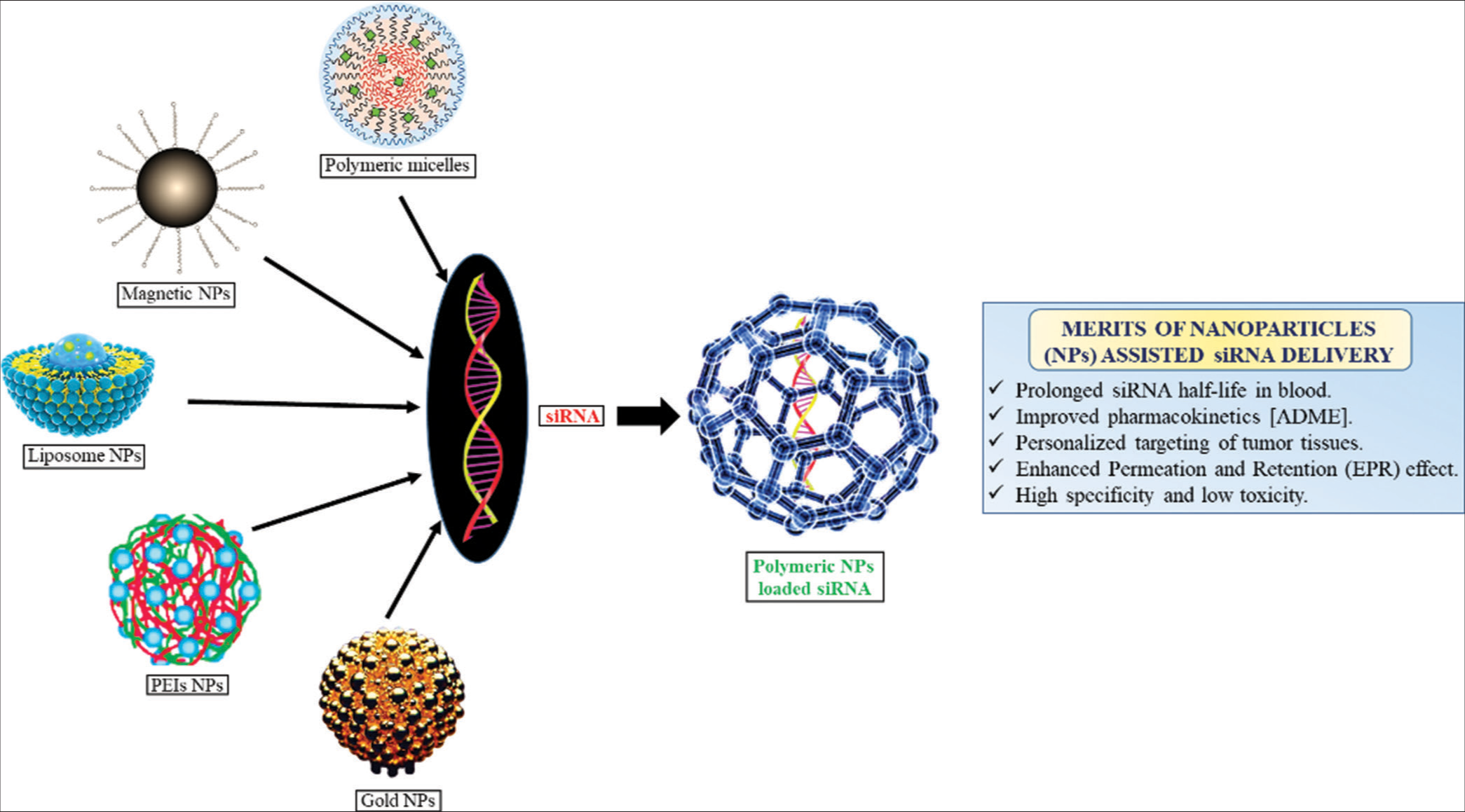
- Challenges for siRNA-based drug delivery. siRNA: Small interfering ribonucleic acid, ADME: Absorption, distribution, metabolism excretion, EPR: Enhanced permeation and retention, PEI: Polyethylenimine.
ROLE OF NANOTECHNOLOGY IN ADDRESSING siRNA-LOADED DRUG DELIVERY CHALLENGES
The successful clinical translation of siRNA therapeutics relies heavily on overcoming several key challenges, particularly related to efficient delivery to target cells and tissues. Nanotechnology offers a promising solution to these challenges by providing innovative platforms for the delivery of siRNA molecules.[53] The rationale for utilizing nanotechnology for siRNA delivery is multifaceted and includes the following considerations:
Enhanced stability and protection
Freestanding siRNA molecules are quickly removed from the bloodstream and are degraded by nucleases in bodily fluids. Encasing siRNA molecules in nanoparticles, polymeric nanoparticles, or viral vectors are examples of nanotechnology-based delivery strategies that will shield the molecules from enzymatic degradation and prolong their half-life in vivo. This enhanced stability increases the likelihood of siRNA reaching target cells intact and active, thereby improving therapeutic efficacy.
Improved pharmacokinetics and biodistribution
Nanoparticle-based delivery systems may modulate the pharmacokinetic properties and biodistribution of siRNA molecules, allowing for controlled release and targeted accumulation in specific tissues or cells. The addition of ligands or targeting moieties to the surface of nanoparticles would enable active targeting of sick tissues or cells and enhance the accumulation of siRNA at the desired site of action while minimizing off-target effects.
Facilitated cellular uptake
Nanoparticles may overcome cellular barriers and facilitate the efficient uptake of siRNA into target cells. Nanoparticle formulations may exploit the endocytic pathways for cellular internalization, leading to higher intracellular delivery of siRNA payloads. Further, improving cellular uptake and intracellular trafficking could be achieved by surface modifications such as the inclusion of cell-penetrating peptides or targeted ligands on nanoparticles loaded with siRNA.
Controlled release and sustained activity
Nanotechnology-based delivery systems may be designed to execute regulated release kinetics and long-lasting siRNA activity. Modulating the physicochemical parameters such as size, surface charge, and composition of nanoparticles would allow scientists to customize the release profile of siRNA payloads to fit the intended therapeutic window. This controlled release ensures prolonged exposure of target cells to therapeutic siRNA concentrations, maximizing the efficacy of gene silencing and therapeutic outcomes.
Minimization of immunogenicity and toxicity
Nanoparticle-based delivery systems should minimize the immunogenicity and toxicity associated with siRNA therapeutics. Biocompatible and biodegradable nanoparticle formulations are designed to minimize adverse immune reactions and cytotoxic effects, making them suitable for repeated administration and long-term therapeutic applications. Furthermore, the rational design of nanoparticles allows for precise control over physicochemical properties to optimize safety profiles and minimize off-target effects.
Scalability and manufacturing considerations
Nanotechnology-based delivery systems offer scalability and manufacturability, facilitating the translation of siRNA therapeutics from bench to bedside. Many nanoparticle formulations may be produced using scalable manufacturing processes, such as nanoprecipitation or microfluidic techniques, enabling efficient production at commercial scales. This scalability is crucial for ensuring the cost-effectiveness and accessibility of siRNA-based therapies for widespread clinical use.[54]
CLINICAL ADVANCEMENTS OF siRNA DELIVERY IN REPRODUCTIVE TRACT CANCERS
According to the present study, nanotechnology-assisted drug delivery to patients suffering from reproductive tract cancers instills confidence in imparting the therapeutic benefits, including the ability to cross the barriers (cellular and immunological) and perform the targeted response, thereby enhancing efficacy and safety of the formulations. Various clinically used siRNA-based formulations for the treatment of reproductive tract cancer are present in Table 1.
| S. No. | siRNA drug | Formulation | Cellular targets | Diseases | References |
|---|---|---|---|---|---|
| 1. | siRNA EphA2-DOPC | DOPC liposome | Ephrin type-A receptor 2 gene | Ovarian cancer | [7] |
| 2. | miR 124-DOPC | DOPC-nanoparticles | p27/myc/phospho-Rb protein | Breast and ovarian cancer | [11] |
| 3. | Anti-miR-451a | PEI based nanoparticle | miR451a | Endometriosis | [29] |
| 4. | Anti-CA 125 antibody | Silica-coated gold particle | CA-125 Antigen | Prostate and ovarian cancer | [50] |
| 5. | PSMA-targeted MNP | Magnetic nanoparticle | PSMA | Prostate cancer detection | [53] |
DOPC: Dioleoylphosphatidylcholine, PSMA: Prostate-specific membrane antigen, MNP: Magnetic nanoparticles, CA: Cancer antigen, PEI: Polyethylenimine.
CONCLUSION
A good and healthy reproductive life is a matter of utmost importance, and it plays a major role in the smooth livelihood of a family with a stress-free environment. Good reproductive health is an achievement for the individual as well as the well-being and development of society together. Despite great healthcare technology and medicine availability from various freebies with the support of the government, reproductive health is still a big stigma and taboo that has seen very modest benefits from. The livelihood with the internet and artificial intelligence is no longer a future endeavor but it has become a present scenario now. Therefore, the involvement of nanotechnology in day-to-day life is not a big surprise, including its involvement in the health sector. Nanotechnology in the reproductive health sector indeed became a revolutionary whirl. The use of remarkable and new strategical nanotechnology-assisted genetic molecules, that is, siRNA has been shown to play a revolutionary role in the near future. It will play a major role in reproductive healthcare for both genders. It should become an important biomarker for reproductive diseases. This article has highlighted the importance of the siRNA and its mechanism for treating reproductive diseases. The techniques for the treatment of ovarian cancer using siRNA-conjugated nanosystems include knock-out of oncogenes, down regulations of genes responsible for metastasis, and drug resistance. It has further discussed the possible strategies for the siRNA-conjugated drug delivery to a specific and desired region of the body through the involvement of nano-based carriers. Concluding that nanotechnology-based delivery systems could offer a rational and versatile approach for overcoming the key challenges associated with siRNA therapeutics, including stability, cellular uptake, targeted delivery, controlled release, and safety. By harnessing the unique properties of nanoparticles, thereby creating novel approaches to facilitate the efficient and successful shipment of siRNA molecules, researchers could maximize the therapeutic promise of RNAi for a range of illnesses.
Challenges and future perspectives
Current challenges in the treatment options and outcomes for malignancies of the reproductive tract, such as endometrial, cervical, and ovarian cancers, are as follows:
Late diagnosis
Reproductive tract malignancies often become apparent at advanced stages, after the disease has progressed from the initial stage. Late diagnosis limits treatment options and reduces the likelihood of successful outcomes.
Limited screening tools
Screening for reproductive tract cancers, particularly ovarian and endometrial cancers, faces challenges due to the lack of highly effective screening tools. Pap smears have significantly reduced cervical cancer incidence, but there is still room for improvement in detecting precancerous lesions and early-stage disease for other cancers.
Heterogeneity of tumors
Reproductive tract cancers exhibit considerable heterogeneity in terms of molecular subtypes, genetic mutations, and clinical behavior. Tailoring treatments to the specific characteristics of individual tumors is crucial for optimizing therapeutic outcomes but presents challenges in developing personalized treatment approaches.
Drug resistance
Resistance to chemotherapy and targeted therapies remain a significant challenge in the treatment of reproductive tract cancers. Cancerous cells can develop mechanisms to evade or resist treatment, leading to treatment failure and disease recurrence.
Toxicity and side effects
Conventional treatments for reproductive tract cancers, such as surgery, chemotherapy, and radiation therapy, may cause significant toxicity and adverse side effects. Managing treatment-related side effects while maintaining therapeutic efficacy is essential for improving patients’ quality of life during and after treatment.
Lack of targeted therapies
While targeted therapies have transformed the treatment landscape for many cancers, there is a need for more targeted treatment options for reproductive tract cancers. Identifying druggable molecular targets and developing effective targeted therapies tailored to the molecular profiles of these cancers is an ongoing challenge.
Immunosuppressive tumor microenvironment
Cancers of the reproductive tract could produce an immunosuppressive tumor microenvironment, which makes it possible for cancer cells to elude immune surveillance and immune-mediated elimination. Overcoming immunosuppression and enhancing antitumor immune responses present challenges in developing effective immunotherapy strategies for these cancers.
Recurrence and metastasis
Despite initial treatment success, reproductive tract cancers may recur and metastasize, leading to poor long-term prognosis. Understanding the mechanisms underlying tumor recurrence and metastasis, thereby developing strategies to prevent or target metastatic spread, is critical for improving survival outcomes.
Addressing these challenges requires interdisciplinary collaboration, innovative research approaches, and an approach to individualized medication that is specific to the features of each patient’s cancer. Advances in precision medicine, targeted therapies, immunotherapy, and early detection technologies hold promise for overcoming these challenges and improving outcomes for individuals affected by reproductive tract cancers.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Epidemiological trends and risk factors of gynecological cancers: An update. Med Oncol. 2023;40:93.
- [CrossRef] [PubMed] [Google Scholar]
- Know about gynaecological cancers and their prevalence in northern parts of India. 2018. Available from: https://www.americanoncology.com/blogs/know-about-gynaecological-cancers-and-their-prevalence-in-northern-parts-of-india#:~:text=every%20year%20in%20india%2c%20122%2c844,from%2087.8%25%20to%2096.67%25 [Last accessed on 2024 Apr 15]
- [Google Scholar]
- Cancer statistics 2024: All hands on deck. CA Cancer J Clin. 2024;74:8-9.
- [CrossRef] [PubMed] [Google Scholar]
- The utilization of nanotechnology in the female reproductive system and related disorders. Heliyon. 2024;10:e25477.
- [CrossRef] [PubMed] [Google Scholar]
- Cervical cancer screening in rural India: Status and current concepts. Indian J Med Res. 2018;148:687-96.
- [CrossRef] [PubMed] [Google Scholar]
- RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6:24560-70.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular landscape and targeted therapy of acute myeloid leukemia. Biomark Res. 2018;6:32.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer biomarkers for targeted therapy. Biomark Res. 2019;7:25.
- [CrossRef] [PubMed] [Google Scholar]
- The role of targeted therapy and biomarkers in breast cancer treatment. Clin Exp Metastasis. 2012;29:807-19.
- [CrossRef] [PubMed] [Google Scholar]
- Functional proteomics identifies miRNAs to target a p27/Myc/phospho-Rb signature in breast and ovarian cancer. Oncogene. 2016;35:691-701.
- [CrossRef] [Google Scholar]
- Overview of benign and malignant tumors of female genital tract. J Appl Pharm Sci. 2013;3:140-9.
- [Google Scholar]
- Predictive and prognostic biomarkers in female genital tract tumors: An update highlighting their clinical relevance and practical issues. Pathology. 2024;56:214-27.
- [CrossRef] [PubMed] [Google Scholar]
- Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15:668-79.
- [CrossRef] [PubMed] [Google Scholar]
- Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382-92.
- [CrossRef] [PubMed] [Google Scholar]
- Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-84.
- [CrossRef] [PubMed] [Google Scholar]
- Maintenance of olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-505.
- [CrossRef] [PubMed] [Google Scholar]
- Oncogenic human papillomaviruses. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160273.
- [CrossRef] [PubMed] [Google Scholar]
- Society for Immunotherapy of Cancer (SITC) clinical practice guidelines on immunotherapy for the treatment of gynecologic cancer. J Immunother Cancer. 2023;11:e006624.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 interpretation in cervical carcinomas: Proceedings of the ISGyP companion society session at the 2020 USCAP annual meeting. Int J Gynecol Pathol. 2021;40:1-4.
- [CrossRef] [PubMed] [Google Scholar]
- ESGO/ESTRO/ESP guidelines for the management of patients with cervical cancer-update 2023. Int J Gynecol Cancer. 2023;33:649-66.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular classification in endometrial cancer: Opportunities for precision oncology in a changing landscape. Cancer. 2022;128:2853-7.
- [CrossRef] [PubMed] [Google Scholar]
- FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162:383-94.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical challenges associated with universal screening for Lynch syndrome-associated endometrial cancer. Cancer Prev Res (Phila). 2017;10:108-15.
- [CrossRef] [PubMed] [Google Scholar]
- Precursor lesions of vulvar squamous cell carcinoma-histology and biomarkers: A systematic review. Crit Rev Oncol Hematol. 2020;147:102866.
- [CrossRef] [PubMed] [Google Scholar]
- Vulvar cancer sub-classification by HPV and p53 status results in three clinically distinct subtypes. Gynecol Oncol. 2020;159:649-56.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of vulvar squamous cell carcinoma and precursor lesions by p16 and p53 immunohistochemistry: Considerations, caveats, and an algorithmic approach. Mod Pathol. 2023;36:100145.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of human papillomavirus and its prognostic value in vulvar cancer: A systematic review and meta-analysis. PLoS One. 2018;13:e0204162.
- [CrossRef] [PubMed] [Google Scholar]
- miR-451a inhibition reduces established endometriosis lesions in mice. Reprod Sci. 2019;26:1506-11.
- [CrossRef] [PubMed] [Google Scholar]
- Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806-11.
- [CrossRef] [PubMed] [Google Scholar]
- Altered gene expression in plants due to trans interactions between homologous genes. Trends Biotechnol. 1990;8:340-4.
- [CrossRef] [PubMed] [Google Scholar]
- RNA as a target of double-stranded RNA-mediated genetic interference in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1998;95:15502-7.
- [CrossRef] [PubMed] [Google Scholar]
- Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494-8.
- [CrossRef] [PubMed] [Google Scholar]
- RNA interference: Biology, mechanism, and applications in cervical cancer. Int J Biol Sci. 2019;15:298-309.
- [Google Scholar]
- Knocking down barriers: Advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129-38.
- [CrossRef] [PubMed] [Google Scholar]
- Epigenetics and genetics in endometrial cancer: New carcinogenic mechanisms and relationship with clinical practice. Epigenomics. 2012;4:147-62.
- [CrossRef] [PubMed] [Google Scholar]
- Investigational IGF1R inhibitors in early stage clinical trial for cancer therapy. Expert Opin Investig Drugs. 2019;28:1101-12.
- [CrossRef] [PubMed] [Google Scholar]
- Nanocarriers for delivery of siRNA and co-delivery of siRNA and other therapeutic agents. Nanomedicine (Lond). 2015;10:2199-228.
- [CrossRef] [PubMed] [Google Scholar]
- Nanoengineered strategies for siRNA delivery: From target assessment to cancer therapeutic efficacy. Drug Deliv Transl Res. 2017;7:346-58.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proc Natl Acad Sci USA. 2011;108:12372-7.
- [CrossRef] [PubMed] [Google Scholar]
- Development of siRNA therapeutics for cancer. Drug Drug Deliv Transl Res. 2010;61:230-48.
- [Google Scholar]
- Advances in the delivery of RNA therapeutics: From concept to clinical reality. Genome Med. 2017;9:60.
- [CrossRef] [PubMed] [Google Scholar]
- Nanotechnology-based strategies for siRNA brain delivery for disease therapy. Trends Biotechnol. 2018;36:562-75.
- [CrossRef] [PubMed] [Google Scholar]
- The business of RNAi therapeutics in 2012. Mol Ther Nucleic Acids. 2012;1:e8.
- [CrossRef] [PubMed] [Google Scholar]
- RNA-based medicine: From molecular mechanisms to therapy. EMBO J. 2023;42:e114760.
- [CrossRef] [PubMed] [Google Scholar]
- Targeted therapies in gynaecologic cancers. Curr Cancer Drug Targets. 2006;6:333-63.
- [CrossRef] [PubMed] [Google Scholar]
- GnRH receptors in cancer: From cell biology to novel targeted therapeutic strategies. Endocr Rev. 2012;33:784-811.
- [CrossRef] [PubMed] [Google Scholar]
- Novel targeted therapies in ovarian and uterine carcinosarcomas. Discov Med. 2018;25:309-19.
- [Google Scholar]
- An ultra-sensitive impedimetric immunosensor for detection of the serum oncomarker CA-125 in ovarian cancer patients. Nanoscale. 2015;7:3768-79.
- [CrossRef] [PubMed] [Google Scholar]
- Nanotechnology for in vivo targeted siRNA delivery. Adv Genet. 2014;88:37-69.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor target amplification: Implications for nano drug delivery systems. J Control Release. 2018;275:142-61.
- [CrossRef] [PubMed] [Google Scholar]
- MRI assessment of prostate-specific membrane antigen (PSMA) targeting by a PSMA-targeted magnetic nanoparticle: Potential for image-guided therapy. Mol Pharm. 2019;16:2060-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacokinetics aspects of biotechnological products In: Biopharmaceutics and pharmacokinetics considerations. Vol 1. United States: Academic Press; 2021. p. :539-65.
- [CrossRef] [Google Scholar]






