Translate this page into:
Sperm a cell in distress: Yoga to the rescue

*Corresponding author: Rima Dada, Department of Anatomy, Lab for Molecular Reproduction and Genetics, All India Institute of Medical Sciences, New Delhi, India. rimadadaaiims20@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dhawan V, Kumar R, Malhotra N, Dadhwal V, Borthakur D, Dada R. Sperm a cell in distress: Yoga to the rescue. J Reprod Healthc Med 2021;2:3.
Abstract
Sperm, one of the complex cells of biological inheritance, are not only considered as mere vectors of transmission of paternal genome at the time of fertilization but also to events post-fertilization. The contribution of sperm molecular factors as a critical determinant of optimal embryonic development and pregnancy outcomes has been brought to surface. Spermatozoa with derangements in redox status, nuclear and mitochondrial genomic integrity, and dysregulated gene expression may affect the fertility status of the male and may result in impaired embryonic development and increase risk of genetic and epigenetic diseases in offspring. The integration of yoga-based lifestyle (YBL) as a part of the modern lifestyle has been found to be beneficial in the management of the derangements in the male reproductive functions in the distressing issue of infertility and early pregnancy loss (EPL) patients. As infertility and EPL are issues with a strong psychosomatic component, yoga a mind body intervention may be a useful adjunctive therapy in the management of these cases and may not only improve the sperm quality but also positively impact the reproductive potential, embryogenesis, and the health of the progeny. This short communication intends to highlight the importance of paternal factors as a causal factor for infertility, EPL, and also the promising role of integration of YBL in the management of such disorders.
Keywords
Sperm
Oxidative stress
DNA damage
Gene expression
Yoga
Meditation
INTRODUCTION
Human sperm cells are highly specialized but yet complex cells when compared to the somatic cells of the body.[1] While earlier, spermatozoa were considered to be vectors of the paternal genome, it is established that reproductive success is largely dependent on the sperm genomic integrity for optimal early embryonic development and health of the offspring.[2,3] Disruptions in sperm DNA integrity are linked to reproductive failures both in spontaneous and assisted conceptions.[2,3] Sperm dysfunction is thus a major contributor to poor fertility. With the availability of genomic information, it has been established that spermatozoa contribute to many processes after fertilization and contribute to the reproductive outcome.[2] Further, abnormal spermatozoa also increase the risk of congenital malformations including dominant genetic disorders as well as neuropsychiatric disorders.[3-5] The vulnerability of the spermatozoa is owing to its being transcriptionally inert, containing minimal cytoplasmic antioxidants, high concentration of polyunsaturated fatty acids (PUFA) in plasma membrane, and lastly being deficient in both the detection and repair of DNA damage.[2-4] Despite the increasing awareness about the role of paternal factors and its contribution toward fertilization and early embryonic development, the advances in diagnosis and treatment still seem to be brought to surface. It thus becomes imperative to understand the various nuances of the structure of the sperm genome and the pitfalls in the various causal factors responsible for the derangements of sperm structure, function, and effects on its reproductive potential and the methods used for alleviation of the same. The advent of a structured yoga-based intervention and its potential role in management needs to be addressed.
SPERM GENOME
Mammalian sperm chromatin undergoes a remarkable level of compaction from a relatively large genome to a very small volume during spermatogenesis. This is achieved by the substitution of the usual nucleosome-based DNA packaging which is present in all somatic cells and premeiotic spermatocytes with a highly compacted sperm-specific nucleoprotamine structure. The compaction of the 145 bp packaging in histone-bound sperm DNA to 50 kbp basic packaging in protamine in doughnut-shaped toroidal structure favors higher efficiency provided due to DNA compaction.[1-3]
Protamines provide compaction of genetic material that protects the sperm genome from both external and internal insults. Protamines replace ~85% of histones in the sperm in a stepwise manner forming the central nucleoprotamine compartment and the rest (~15%) remains bound to nucleohistones in the sperm periphery.[1-3] This retention of nucleosomes in the peripheral compartment of sperm genome includes telomeres and promoters of genes regulating early embryonic development. The peripheral nucleohistone compartment is vulnerable to oxidative stress (OS) and also aids in the maintenance of the genomic imprints.[1-3] Defects in sperm chromatin compaction and concomitant increase in sperm histone:protamine ratio predisposes to an increased risk of male infertility.[6] Oxidative insult resulting in lipid peroxidation and DNA damage significantly affects the spermatozoa, despite their tightly compacted chromatin.[4,5,7,8] Oxidative DNA damage (ODD) is a deleterious consequence of cellular metabolism as it shows a tremendous increase following any toxic insult.
OS
OS exists when the number of oxidants exceed the antioxidants due to either an excessive generation of reactive oxygen species (free radicals) or their inadequate scavenging by antioxidants. This results in homeostatic imbalance associated with cellular damage. Reactive oxygen species (ROS), are highly reactive oxidizing agents generated as a result of oxygen metabolism which are rendered reactive due to the presence of unpaired electrons. These include superoxide anion (O2.), hydrogen peroxide (H2O2), peroxyl (ROO.), and hydroxyl (OH.) radicals.[4,5,9-12] The production of ROS have been considered as a major byproduct of sperm metabolism. The resultant generation of these free radicals is essential in the physiological regulation of several physiological processes in the spermatozoa including capacitation, hyperactivation, acrosome reaction, fusion of sperm with the oocyte, and activation of signal transduction pathways.[2,8,13] Pathological levels of ROS are responsible for induction of sperm DNA damage and adversely impair the fertilizing potential.[5,14,15] Generation of ROS can occur from both endogenous and exogenous sources. A number of endogenous processes such as cytochrome P450-catalysed drug metabolism, oxidative phosphorylation, and inflammatory cell activation can also result in the generation of ROS.
Causes and effects
The vulnerability of the sperm to damage due to oxidative insult is the result of sperm being transcriptionally inactive due to compaction, deficient in cytosolic antioxidants, a high content of PUFA content in cell membrane, and inadequate DNA damage detection and repair mechanisms (base excision repair mechanism).[3,4,7,15-18] There are numerous lipid metabolites which are generated as a consequence of the oxidative attack by free radicals on the PUFA in the sperm membrane including lipid alkoxyl radicals, peroxyl radicals, and also various electrophiles, such as 4-hydroxynonenal (4HNE), malondialdehyde (MDA), and acrolein.[15,16] These electrophillic aldehydes have the potential to form adducts with several proteins in spermatozoa affecting sperm function. Moazamian et al. showed the formation of an adduct formed with the flagellar axonemal protein, dynein heavy chain in their study, which highlighted the impact on sperm motility.[15] Several proposed factors which induce seminal OS, include morphologically abnormal sperm, sedentary lifestyle, intake of nutritionally depleted food, infections, exposure to xenobiotics, psychological stress, high temperature, insecticides and pesticides, smoking, alcohol, obesity, mobile phone radiations, and advanced paternal age.[7] This makes the spermatozoa susceptible to OS-induced damage to various biomolecules and to sperm mitochondrial and nuclear genome. The excess production of ROS by such pathways is detrimental for the sperm functions and acts by two mechanisms, (a) by producing lipid oxidation in the sperm plasma membrane of the sperm containing bountiful PUFAs making them susceptible to oxidative attack and (b) by affecting the sperm mitochondrial and nuclear DNA as well as RNA, thus further compromising embryonic development. The highly complex lipid peroxidation pathway, electrophilic aldehydes which further increases the production of OS.[8,14,19] This is due to inherent potential of aldehydes to covalently bind to the nucleophilic centers of vulnerable proteins, in the mitochondrial electron transport chain (ETC) such as succinic acid dehydrogenase.[8,20] The subsequent disruption of mitochondrial electron transport, leads to an electron efflux which become associated with oxygen, generating superoxide anion.[8] This culminates in a self-perpetuating redox cycle and any factor predisposing to OS will lead to greater levels of oxidative damage, along with accelerated generation of mitochondrial ROS and a state of cell senescence, as these cells thus enter a truncated version of the intrinsic apoptotic cascade.[5,21] One of the first functions affected by OS and lipid peroxidation is sperm motility.[22,23] It has been proposed that oxidative damage to the axoneme and depletion of intracellular adenosine triphosphate (ATP) are responsible for asthenospermia which is one of the first manifestations of OS and lipid peroxidation. Shamsi et al. documented that OS and OS-induced mitochondrial dysfunction are associated with reduced ATP levels which impair development and differentiation of microtubular apparatus of sperm axoneme and results in asthenozoospermia.[14]
The vulnerable spermatozoa are endowed by a repertoire of antioxidant scavenging mechanisms to safeguard from the ROS attack. Taking into account that spermatozoa are transcriptionally inactive and possess limited endogenous antioxidant defense mechanisms, the dependence on exogenous source of antioxidants has mushroomed among reproductive specialties.[24,25] This fact has given rise to the adoption of different cocktails of enzymatic and non-enzymatic antioxidants which are currently used therapeutically. Most of the antioxidants used lack consistency and also the question that what defines the ideal redox balance remains unanswered.[26,27] Adoption of a healthy yoga-based lifestyle (YBL) has not only seen to show improvement in decreasing ROS levels but also regulating levels of free radicals.
SPERM (NUCLEAR AND MITOCHONDRIAL) GENOMIC INTEGRITY
Nuclear genome
The high level of derangements in the nuclear chromatin in the male germ line is one of the hallmarks of male infertility. The compromise in the sperm genomic integrity has been found to be clinically associated with an array of adverse reproductive outcomes including impaired fertilization, aberrant embryogenesis, an increased rate of spontaneous miscarriage, implantation failure, congenital malformations, and significant morbidity in the offspring, including paediatric carcinomas.[5,28] The detailed etiology of the defects in genomic integrity are still underexplored but they have been associated with efficiency of sperm chromatin compaction during the terminal stages of sperm differentiation in epididymis and oxidative insult on the spermatozoa.[5,20,29,30] The guanine base in the sperm chromatin is more susceptible to the oxidative attack on the sperm due to its low oxidation potential. Due to the lack of adequate repair mechanisms in sperm, the impending attack results in accumulation of the mutagenic base adduct 8-hydroxy-2-deoxyguanosine (8-OHdG). 8-OHdG expression ahas been shown to correlate with DNA fragmentation on terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, chromatin integrity assays as well as in vivo fertility.[28,31] 8-OHdG has the propensity to form a stable base pair with adenine, resulting in G:C to T:A transversion mutations during DNA replication. This has a highly mutagenic potential if it is able to escape from DNA repair.[2-4,29] It thus becomes imperative that 8-OHdG lesions are removed from DNA-damaged spermatozoa to prevent it from affecting the early embryonic gene expression. OS and associated oxidative damage to both mitochondrial and nuclear DNA, shortening of telomeres, and accumulation of mitochondrial DNA (mtDNA) variations and highly mutagenic oxidized DNA adducts also predispose to an increased risk for cancer.[2,3,19,32] The accumulation of the mutagenic base adducts may also lead to an increased risk of various gonadal and extragonadal tmalignancies later in life making male infertility an early marker of such late onset malignancies.[33] OS is also associated with aberrant DNA methylation with global hypomethylation and associated loss of genomic stability may also increase risk for cancer.[3,32] In an attempt to elucidate the paternal contributions in recurrent pregnancy loss (RPL), a study conducted in our laboratory estimated the OS and DNA integrity and significant difference was found between patients and healthy fertile controls. The odds of occurrence of RPL were estimated to be 4.23 time higher when ROS were >27.8 RLU/sec/million sperm (OR 4.23, 95% CI: 1.6–11.1), while it was 10.21 times higher when DFI was >30.7 (OR 10.21, 95% CI: 3.1–33.5). The study also assessed relative expression of the genes critical for early embryonic development.[2] Thus, an intervention which results in decrease in ROS levels, improvement in sperm nuclear DNA integrity, increases in total antioxidant capacity (TAC), and also helps in maintenance of telomere length is ideal for reduction of idiopathic infertility, RPL, congenital malformation, and reduction of disease burden in children. [2,3,33] The lack of adequate detection and repair mechanisms of DNA damage in spermatozoa makes it susceptible to various oxidative insults and thus, it is better to adopt necessary changes to prevent the impending oxidative attack than only treating it later. Thus, it becomes prudent to minimize exposure causal factors inducing OS, and if diagnosed with high levels, then simple lifestyle intervention like yoga may normalize the levels.
Mitochondrial genome
Mitochondria are one of the potential endogenous source of ROS production in spermatozoa. Mitochondrion, present in sperm midpiece, is also the only organelle to have its own genome in the sperm. Despite their location, the mitochondria rely on the glycolytic pathway to meet their energy demands. This extrachromosomal genetic counterpart in the sperm has the capacity to synthesize proteins in a semi-autonomous manner. Human mtDNA circular dsDNA molecule (16569 bp). It is responsible for the coding for 2 rRNAs, 22 tRNAs, and 13 polypeptides.[34-36] mtDNA only possesses the genes, which regulate OXPHOS pathway, and this reduction in genomic material warrants against any scope of error. The mtDNA is susceptible to oxidative attack as it lacks the protection by histones or DNA-binding proteins unlike the genomic DNA and has a very basic repair mechanism.[4,35]
In the study conducted in our laboratory, mitochondrial health was assessed by calculation of mitochondrial copy number variation and transcripts associated with mitochondrial integrity: AMPK, IGF1R, PRC-1, TFAM, SIRT-1, TIMP-1, and CLOTHO.[37] The study showed that there was a significant increase in mitochondrial copy number and increased expression of transcripts that maintain mitochondrial integrity after 12 weeks of intervention.[37] Adoption of yoga-based lifestyle intervention as an integral part of our lifestyle may hold the key to increase mitochondrial copy number, increase the expression levels of transcripts that maintain mitochondrial integrity and its associated consequences on physical, mental, and reproductive health. Though the mitochondria present in sperm are not transmitted to the offspring, however, the mtDNA damage is associated with lower ATP production and higher free radical production which damages all biomolecules and both nuclear and mtDNA. It also results in production of sperm which are poorly differentiated and have impaired motility.[35,36,38,39] Sperm mitochondria are prone to electron leakage, resulting in production of ROS and suppressing the sperm functions. A host of factors might trigger the production of ROS by mitochondria, (a) directly by interfering the passage of electrons to the electron transport chain, for example, polyunsaturated fatty acids,[35] electromagnetic radiation[40] and (b) indirectly by activation of intrinsic apoptotic pathway, for example, bisphenol A or parabens.[41,42]
YOGA- AND MEDITATION-BASED LIFESTYLE INTERVENTION (YBLI)
Previous evidence from literature has suggested that yoga and meditation training exerts a range of salubrious effects, including decline in OS, ODD, regulation of telomere length, telomerase activity, and sperm transcript normalization. Since YBLI comprises of mind and body based relaxation practices for intentional and self-regulated attention focus, its obvious aim would be to pacify mind and relax the body. [43] Stress and anxiety in the current lifestyle have contributed to the pathogenesis of various complex chronic diseases with a concurrent decrease in the quality of life. Though various pharmacological modalities have been adopted for the management of such lifestyle disorders and overwhelming chronic stress but the advent of YBLI as non-pharmacological modality can serve as a promising option for management of such conditions. Regular practice of Sudarshan Kriya and pranayama had been associated with stress reduction and in improving the functions of immune system.[44] Various biochemical markers can be used to quantify psychological stress such as cortisol, β-endorphins, IL-6, and TNF-α. Regular adoption of yoga and meditation has also been seen to affect the levels of various hormones affecting the biological functions such as growth hormone, prolactin, melatonin, corticotrophin-releasing hormone, glucocorticoids, thyroid hormone, and other neurotransmitters such as endorphins, serotonin, 5-hydroxy indole acetic acid, DHEAS (dehydroepiandrosterone), gamma amino butyric acid, and 8-OHdG.[45-52] Yoga and meditation showed reduction in psychological stress as well as improvement (reduction of seminal OS and ODD) in sperm derangements[53-57] and quality of life.[58-61] Various researchers have previously assessed the impact of yoga on OS in various conditions.[62-64] Impact of adoption of Hatha yoga was assessed by Gordon et al., in their study evaluated OS markers patients with end-stage renal disease who were on hemodialysis.
Adoption of YBLI for a short-term in a randomized controlled trial conducted in our laboratory documented a decrease in clinical severity in major depressive disorder patients. The study witnessed an increase in neuroplasticity along with an increase in levels of sirtuin 1, DHEAS, telomerase activity, and a decrease in the levels of cortisol, interleukin 6 and also a decrease in ODD and regulation of ROS levels.[65]
Impact on OS and ODD
Various studies previously conducted in our laboratory have reported a rapid significant decline in seminal OS even within just 10 days of regular practice of yoga and meditation.[54-56,66,67] Dhawan et al., 2018a,b, reported a minimal non-significant improvement in the DNA fragmentation index measured to assess the ODD in male partners of couples with recurrent pregnancy loss and implantation failure in IVF cycles.[55,56] While the significant improvement in ODD took 6 months as reported by decrease in levels of mutagenic base adduct 8-OHdG[32,53,54] and an increase in seminal total antioxidant capacity (TAC) levels. [32,54] The decline in levels of this adduct is important as it can be a crucial causal factor of infertility, reproductive outcomes, and childhood disease burden.[31,32,51,55,56] OS has shown to exert a biphasic response, as it is beneficial in low levels and deleterious in higher levels. Moderate levels of OS have been cited to be beneficial in maintenance of sperm telomere length in another study conducted in our laboratory whereas very high and low levels of seminal OS have been associated with shorter telomere length and decline in DNA integrity.[18] Telomere length has been indicated as one of the most relevant indicators of sperm function. The sperm telomere length in male partners of females with RPL has been found to be significantly lower as compared to that seen in healthy fertile controls in the recent study from our laboratory (unpublished). The study also documented a significant positive increase in the relative sperm telomere length with the adoption of YBL intervention by the couples. It has also been cited to be a marker of psychological stress and aging.[65,68,69] The integration of a healthy YBL intervention has seen to not only affect the telomere length but also another important marker of sperm biology, that is, the telomerase activity. Increase in telomerase activity along with decrease in ROS levels and increase in TAC levels were witnessed by a short-term (21 days) practice of YBLI. Yoga and meditation based lifestyle has proven to be therapeutic for sperm and the effects on sperm can be summarized in [Figure 1].[54]
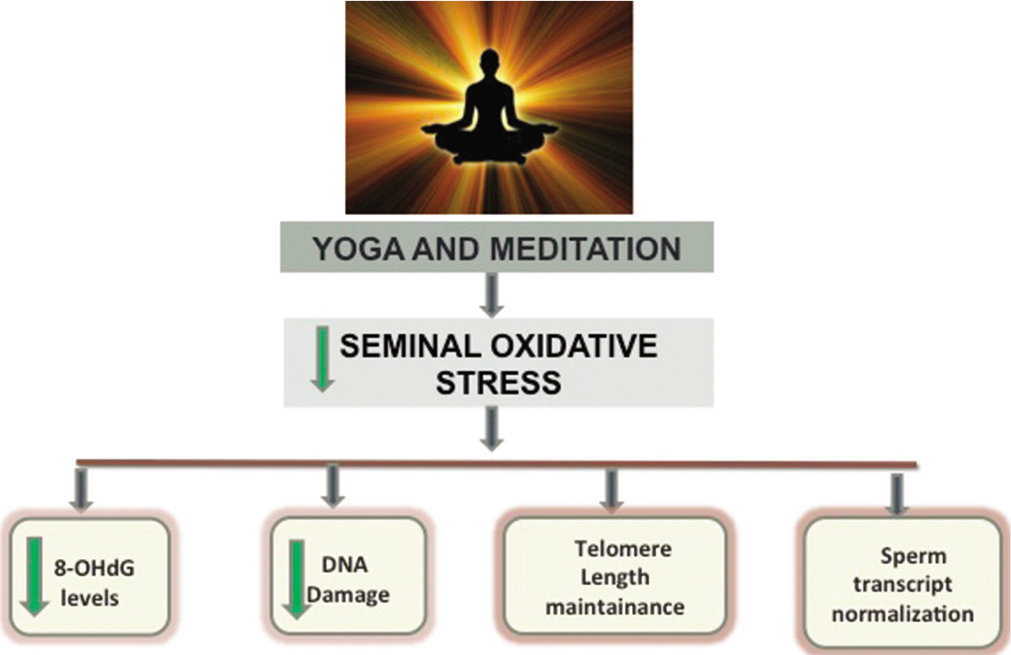
- Effect of yoga and meditation based lifestyle intervention on the sperm.
Yoga versus antioxidants
The therapeutic use of various enzymatic and nonenzymatic antioxidant formulations is being routinely adopted by specialists in reproductive medicine for alleviating the current burden of derangements due to OS and ODD.[70] Different antioxidant formulations have been proposed to show improvement in oligoasthenospermia but therapeutic doses have seen to affect DNA damage in only a few studies.[8,70] Yoga-based interventions have been significantly seen to impact ROS levels with a parallel increase in antioxidant levels (TAC levels) in as early as 10 days.[32,33,57,71-73] They have been documented to regulate rather than simply scavenging reactive oxygen species. Lack of consensus, dosage schedules and indiscriminate use of antioxidants among practitioners causes very low levels of free radicals resulting in impaired sperm function and several redox sensitive metabolic reactions. YBLI has not only seen to mediate regulation of ROS levels but also cause an alleviation and reversal of various mechanisms causing stress. It has been shown to bring about positive changes by moderating OS-related gene expression[74-80] immediately by parasympathetic activation, decrease in pro-oxidants,[81] and increase in antioxidants (like melatonin).[51,82]
Effect on gene expression
YBLI does not have short term effect only, but it has also shown long term effects by causing histone modifications.[83] Spermatozoal transcripts, with a critical role in early embryogenesis showed normalization in the levels in male partners of couples with history of recurrent pregnancy loss and implantation failure by Dhawan et al., (2018) after adopting a brief YBLI for 21 days.[55,56] The dysregulation in gene expression showed normalization toward that of healthy fertile controls with this brief intervention.[55,56] In the study from our laboratory on primary open angle glaucoma patients, we found positive changes in gene expression pattern by using whole genome microarrays.[61,66] An upregulation of genes involved in cellular repair and nerve growth maintenance and genes was observed in our patient cohort[60,61,84] in the central as well as peripheral tissues. The multisystem effects observed by integration of yoga and meditation is thus ideal in management of infertility and reversing testicular aging. A pilot study conducted in our laboratory to assess the changes in the sperm epigenome of male infertility patients by next-generation sequencing documented alterations in the sperm methylome following yoga-based intervention for 21 days. This was seen as DNA methylation changes at 400 genes, out of which hypermethylation was seen in 147 genes and hypomethylation of 229 genes was found.[85]
CONCLUSION
OS in spermatozoa has been highlighted as one of the root causes of underlying defects in the sperm cell and also an important determinant of sperm genomic stability as well as reproductive potential. The integration of YBLI is of immense relevance as even hough the various etiologies of infertility, pregnancy loss and birth defects involving genetic factors can not be reversed but an attempt can be made to minimize the ODD by adopting a healthy lifestyle. Yoga- and meditation-based lifestyle has been proven to be therapeutic for sperm functions as they lower seminal OS, improve genomic integrity, decrease the mutagenic load in sperm DNA, and aid in telomere length maintenance and normalization of sperm transcript levels. This may also further aid in reducing the number of couples who have to resort to assisted reproduction for conception and even help in reducing incidence of pregnancy losses, implantation failures and significant morbidity and mortality in the offspring. Thus, this positive impact on the sperm epigenome by yoga may help in reducing the rate of testicular aging and incidence of various genetic and epigenetic diseases in the offspring.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Paternal DNA packaging in spermatozoa: More than the sum of its parts? DNA histones, protamines and epigenetics. Reproduction. 2010;139:287-301.
- [CrossRef] [PubMed] [Google Scholar]
- Paternal factors and embryonic development: Role in recurrent pregnancy loss. Andrologia. 2019;51:e13171.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of sperm molecular factors, oxidative damage and transcripts in childhood disorders. J Child Dev Disord. 2017;3:6.
- [CrossRef] [Google Scholar]
- Oxidative stress and male infertility. Nat Rev Urol. 2017;14:470-85.
- [CrossRef] [PubMed] [Google Scholar]
- Biological and clinical significance of DNA damage in the male germ line. Int J Androl. 2009;32:46-56.
- [CrossRef] [PubMed] [Google Scholar]
- Sperm nuclear histone to protamine ratio in fertile and infertile men: Evidence of heterogeneous subpopulations of spermatozoa in the ejaculate. J Androl. 2006;27:414-20.
- [CrossRef] [PubMed] [Google Scholar]
- On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16:3-13.
- [CrossRef] [PubMed] [Google Scholar]
- On methods for the detection of reactive oxygen species generation by human spermatozoa: Analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology. 2013;1:192-205.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of oxidative stress on male and female germ cells: Implications for fertility. Reproduction. 2020;159:R189-201.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: An evidence based analysis. Int Braz J Urol. 2007;33:603-21.
- [CrossRef] [PubMed] [Google Scholar]
- Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. 2016;28:1-10.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance of reactive oxygen species in semen of infertile Indian men. Andrologia. 2009;41:251-6.
- [CrossRef] [PubMed] [Google Scholar]
- Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal. 2011;14:367-81.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of nuclear DNA damage in human spermatozoa in men opting for assisted reproduction. Indian J Med Res. 2008;127:115-23.
- [Google Scholar]
- Oxidative stress and human spermatozoa: Diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol Hum Reprod. 2015;21:502-15.
- [CrossRef] [PubMed] [Google Scholar]
- Defining the mechanisms by which the reactive oxygen species by-product, 4-hydroxynonenal, affects human sperm cell function. Biol Reprod. 2015;92:108.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative damage to sperm DNA: Clinical implications. Andrology. 2014;3:116.
- [CrossRef] [Google Scholar]
- Mild oxidative stress is beneficial for sperm telomere length maintenance. World J Methodol. 2016;6:163-70.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidation of sperm nucleus in mammals: A physiological necessity to some extent with adverse impacts on oocyte and offspring. Antioxidants (Basel). 2020;9:95.
- [CrossRef] [PubMed] [Google Scholar]
- Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol Reprod. 2012;87:110.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod Biomed Online. 2003;7:65-70.
- [CrossRef] [Google Scholar]
- Evaluation of a spectrophotometric assay for the measurement of malondialdehyde and 4-hydroxyalkenals in human spermatozoa: Relationships with semen quality and sperm function. Int J Androl. 1998;21:81-94.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress and male reproductive health. Asian J Androl. 2014;16:31-8.
- [CrossRef] [PubMed] [Google Scholar]
- Yoga, meditation and acupuncture for male reproductive health In: Parekattil SJ, Esteves SC, Agarwal A, eds. Male Infertility: Contemporary Clinical Approaches, Andrology, ART and Antioxidants (2nd ed). Berlin: Springer Nature; 2019. p. :590-602.
- [CrossRef] [Google Scholar]
- Alternative medicine and fertility outcome In: Devi MG. Talwar P, Agarwal A, Mehta J, editors. Textbook of Assisted Reproductive Technology. India: Indian Fertility Society;
- [Google Scholar]
- A novel antioxidant formulation designed to treat male infertility associated with oxidative stress: Promising preclinical evidence from animal models. Hum Reprod. 2016;31:252-62.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular changes induced by oxidative stress that impair human sperm motility. Antioxidants (Basel). 2020;9:134.
- [CrossRef] [PubMed] [Google Scholar]
- Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: Systematic review and meta-analysis. Hum Reprod. 2008;23:2663-8.
- [CrossRef] [PubMed] [Google Scholar]
- DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2'-deoxyguanosine, a marker of oxidative stress. Biol Reprod. 2009;81:517-24.
- [CrossRef] [PubMed] [Google Scholar]
- DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005;322:33-41.
- [CrossRef] [PubMed] [Google Scholar]
- Fertilization stimulates 8-hydroxy-2'-deoxyguanosine repair and antioxidant activity to prevent mutagenesis in the embryo. Dev Biol. 2015;406:1-13.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress induced damage to paternal genome and impact of meditation and yoga-can it reduce incidence of childhood cancer? Asian Pac J Cancer Prev. 2016;17:4517-25.
- [Google Scholar]
- Male infertility: A biomarker of individual and familial cancer risk. Fertil Steril. 2018;109:6-19.
- [CrossRef] [PubMed] [Google Scholar]
- Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457-65.
- [CrossRef] [PubMed] [Google Scholar]
- Mitochondrial DNA mutations in etiopathogenesis of male infertility. Indian J Urol. 2008;24:150-4.
- [CrossRef] [PubMed] [Google Scholar]
- Role of reactive oxygen species in the pathogenesis of mitochondrial DNA (mtDNA) mutations in male infertility. Indian J Med Res. 2009;129:127-37.
- [Google Scholar]
- Improvement in mitochondrial integrity and fertility potential in men with Rheumatoid arthritis by yoga-based lifestyle intervention. Hum Reprod. 2020;35:i168-9.
- [Google Scholar]
- Defective human sperm cells are associated with mitochondrial dysfunction and oxidant production. Biol Reprod. 2015;93:119.
- [CrossRef] [PubMed] [Google Scholar]
- Sperm mitochondrial dysfunction and oxidative stress as possible reasons for isolated asthenozoospermia. J Physiol Pharmacol. 2018;69:403-17.
- [Google Scholar]
- Probing the origins of 1, 800 MHz radio frequency electromagnetic radiation induced damage in mouse immortalized germ cells and spermatozoa in vitro. Front Public Health. 2018;6:270.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro exposure of human spermatozoa to bisphenol a induces pro-oxidative/apoptotic mitochondrial dysfunction. Reprod Toxicol. 2016;66:61-7.
- [CrossRef] [PubMed] [Google Scholar]
- Parabens generate reactive oxygen species in human spermatozoa. Andrology. 2018;6:532-41.
- [CrossRef] [PubMed] [Google Scholar]
- Dorland's Illustrated Medical Dictionary (32nd ed). Philadelphia, PA: Saunders; 2012. p. :2147.
- [Google Scholar]
- Effect of rhythmic breathing (Sudarshan Kriya and Pranayam) on immune functions and tobacco addiction. Ann N Y Acad Sci. 2005;1056:242-52.
- [CrossRef] [PubMed] [Google Scholar]
- Serotonin, noradrenaline, dopamine metabolites in transcendental meditation-technique. J Neural Transm. 1976;39:257-67.
- [CrossRef] [PubMed] [Google Scholar]
- Sympathetic activity and transcendental meditation. J Neural Transm. 1979;44:117-35.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine changes during transcendental meditation. Clin Exp Pharmacol Physiol. 1980;7:75-6.
- [Google Scholar]
- Stress reduction and preventing hypertension: Preliminary support for a psychoneuroendocrine mechanism. J Altern Complement Med. 1995;1:263-83.
- [CrossRef] [PubMed] [Google Scholar]
- Catecholamine levels in practitioners of the transcendental meditation technique. Physiol Behav. 2001;72:141-6.
- [CrossRef] [Google Scholar]
- The effects of long meditation on plasma melatonin and blood serotonin. Med Sci Monit. 2004;10:CR96-101.
- [Google Scholar]
- Impact of integrated amrita meditation technique on adrenaline and cortisol levels in healthy volunteers. Evid Based Complement Alternat Med. 2011;2011:379645.
- [CrossRef] [PubMed] [Google Scholar]
- Yoga and meditation as a therapeutic intervention in oxidative stress and oxidative DNA damage to paternal genome. J Yoga Phys Ther. 2015;5:4.
- [CrossRef] [Google Scholar]
- Telomerase activity and cellular aging might be positively modified by a yoga-based lifestyle intervention. J Altern Complement Med. 2015;21:370-2.
- [CrossRef] [PubMed] [Google Scholar]
- Yoga based lifestyle intervention in the management of recurrent implantation failure. Indian J Sci Res. 2018;18:1-8.
- [Google Scholar]
- Meditation and yoga: Impact on oxidative DNA damage and dysregulated sperm transcripts in male partners of couples with recurrent pregnancy loss. Indian J Med Res. 2018;148:134-9.
- [Google Scholar]
- Mind-body interventions significantly decrease oxidative DNA damage in sperm genome: Clinical implications. React Oxyg Species. 2019;7:1-9.
- [CrossRef] [Google Scholar]
- Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer out patients. Psychosom Med. 2003;65:571-81.
- [CrossRef] [PubMed] [Google Scholar]
- Meditation reduces sympathetic activation and improves the quality of life in elderly patients with optimally treated heart failure: A prospective randomized study. J Altern Complement Med. 2005;11:465-72.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of yoga and meditation based intervention on intraocular pressure, quality of life, oxidative stress, gene expression pattern in primary open angle glaucoma: A randomized controlled trial. Invest Ophthalmol Vis Sci. 2016;57:1.
- [Google Scholar]
- Impact of meditation and yoga based intervention on quality of life and stress markers in glaucoma patients: A prospective randomized controlled study. Int Glaucoma Rev. 2015;16:237.
- [Google Scholar]
- Effect of yoga exercise therapy on oxidative stress indicators with end-stage renal disease on hemodialysis. Int J Yoga. 2013;6:31-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of yoga on oxidative stress in elderly with Grade-I hypertension: A randomized controlled study. J Clin Diagn Res. 2014;8:BC04-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of yoga on oxidative stress, motor function, and non-motor symptoms in Parkinson's disease: A pilot randomized controlled trial. Pilot Feasibility Stud. 2018;4:162.
- [CrossRef] [PubMed] [Google Scholar]
- Yoga and meditation based intervention increases neuroplasticity and reduces severity of major depressive disorder: A randomized controlled trial. Restor Neurol Neurosci. 2018;36:423-42.
- [CrossRef] [PubMed] [Google Scholar]
- Estimation of blood free radical levels in healthy population pre and post yoga. J Anat Soc India. 2014;63:S13-8.
- [CrossRef] [Google Scholar]
- Impact of meditation and yoga on oxidative DNA damage in sperm: Clinical implications. J Yoga Phys Ther. 2016;6:3.
- [CrossRef] [Google Scholar]
- Anticipatory sensitization to repeated stressors: The role of initial cortisol reactivity and meditation/ emotion skills training. Psychoneuroendocrinology. 2015;52:229-38.
- [CrossRef] [PubMed] [Google Scholar]
- Meditation, stress processes, and telomere biology. Curr Opin Psychol. 2018;28:92-101.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665-70.
- [CrossRef] [PubMed] [Google Scholar]
- Role of antioxidants in treatment of male infertility: An overview of the literature. Reprod Biomed Online. 2004;8:616-27.
- [CrossRef] [Google Scholar]
- Sudarshan Kriya practitioners exhibit better antioxidant status and lower blood lactate levels. Biol Psychol. 2003;63:281-91.
- [CrossRef] [Google Scholar]
- Improvement of glutathione and total antioxidant status with yoga. J Altern Complement Med. 2007;13:1085-90.
- [CrossRef] [PubMed] [Google Scholar]
- Mind-body medicine: Effect of the mind on gene expression. Pers Med Univ. 2012;1:2-6.
- [CrossRef] [Google Scholar]
- The role of neurotrophins in brain aging: A perspective in honor of Regino Perez-Polo. Neurochem Res. 2005;30:877-81.
- [CrossRef] [PubMed] [Google Scholar]
- Mindfulness-based stress reduction training reduces loneliness and proinflammatory gene expression in older adults: A small randomized controlled trial. Brain Behav Immun. 2012;26:1095-101.
- [CrossRef] [PubMed] [Google Scholar]
- Genome-wide expression changes in a higher state of consciousness. Conscious Cogn. 2012;21:1322-44.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid gene expression changes in peripheral blood lymphocytes upon practice of a comprehensive yoga program. PLoS One. 2013;8:e619.
- [CrossRef] [PubMed] [Google Scholar]
- What is the molecular signature of mind-body interventions? A systematic review of gene expression changes induced by meditation and related practices. Front Immunol. 2017;8:670.
- [CrossRef] [PubMed] [Google Scholar]
- From cDNA microarrays to high-throughput proteomics. Implications in the search for preventive initiatives to slow the clinical progression of Alzheimer's disease dementia. Restor Neurol Neurosci. 2001;18:137-42.
- [Google Scholar]
- Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013;38:1698-708.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin reduces oxidative stress and improves vascular function in pulmonary hypertensive newborn sheep. J Pineal Res. 2015;58:362-73.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid changes in histone deacetylases and inflammatory gene expression in expert meditators. Psychoneuroendocrinology. 2014;40:96-107.
- [CrossRef] [PubMed] [Google Scholar]
- Yoga and meditation based intervention alters global genome expression profile in primary open angle glaucoma (POAG): Role in oxidative stress management. J Int Soc Antioxid Nutr Health. 2016;3
- [CrossRef] [Google Scholar]
- Sperm methylome alterations following yoga-based lifestyle intervention in patients of primary male infertility: A pilot study. Andrologia. 2020;52:e13551.
- [CrossRef] [PubMed] [Google Scholar]






