Translate this page into:
SOX4 expression in cancer: Insights from developmental regulation and deregulation in tumorigenesis

*Corresponding author: Nirmala Jagadish, Centre for Cancer Immunotherapy, Mahatma Gandhi Medical College and Hospital, Jaipur, Rajasthan, India. nirmalajagadish26@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Arora S, Godha M, Lohiya NK, Jagadish N. SOX4 expression in cancer: Insights from developmental regulation and deregulation in tumorigenesis. J Reprod Healthc Med. 2024;5:5. doi: 10.25259/JRHM_8_2024
Abstract
The burden of cancer is on a rapid rise globally. Deregulated gene expression profiles may lead to the development of cancer. Master regulators are the regulatory checkpoints that define and control the intricate networks of gene and protein interactions that make up cell physiology. The molecular programs that generate particular cellular phenotypes depend on master regulators. SOX4 gene is a master regulator that controls the expression of other genes responsible for tumorigenesis and plays a crucial role in multiple signaling pathways. The expression of SOX4 is upregulated in various malignancies. Increased proliferation of cancer cells, survival, apoptosis, and epithelial-to-mesenchymal transition leading to metastasis have all been linked to SOX4 expression in cancer. Elevated levels of SOX4 also possess a correlation with poor prognosis in various cancer types. Recently, SOX4 has surfaced as a possible target for cancer therapeutics. Furthermore, it has been shown that targeting SOX4 could inhibit tumor growth and enhance the efficacy of conventional cancer therapies. The present review summarizes the current status of SOX4 in the initiation and progression of various human cancers.
Keywords
Cancer
Master regulator
SOX4
Prognosis
Therapeutic
INTRODUCTION
The sex-determining region Y (SRY) gene is located on the Y chromosome and is crucial for the determination of sex-specific fates and the development of male sexuality.[1-3] The SOX gene family comprises 20 members, which are divided into nine subgroups, varying from SOXA to SOXH.[4] A comparison among various members of the SOX family has been illustrated in Table 1.[5-57] SRY-related high-mobility-group-box 4 (SOX4) belongs to the C subfamily of SOX transcription factors and has a significant role in the growth and differentiation of cells and organs during early development. SOX4 is also linked to the initiation and progression of various human malignancies.[4,58] The SOX4 gene is found on chromosome 6p22.3 in humans. It encodes for a 47 KDa protein constituting 474 amino acids and is highly conserved in vertebrates. In addition to being crucial for the development of bones, islets, and the heart, SOX4 also plays a crucial role in cancers, osteoporosis, and other diseases. SOX4 executes its impact on context-specific gene expression regulation and tissue-specific tumorigenesis by interacting with numerous other transcription factors. Several investigations about the medical relevance of SOX4 in cancer suggest its function in tumor growth and development.[59] Increased expression of SOX4 in various human cancers is frequently linked to poor clinical outcomes. SOX4 also mediates several signaling pathways which contribute to tumorigenesis and tumor progression. Several pathways regulated by SOX4 are depicted in Figure 1.
| Subfamily | Member | Chromosomal position |
Physiological function | Role in cancer | References |
|---|---|---|---|---|---|
| Group A | SRY | Yp11.2 | Sex determination | Prognostic biomarkers in head and neck SCC | [5,6] |
| Group B1 | SOX1 | 13q34 | Involved in brain development | SOX1 overexpression inhibits cell proliferation, invasion, and tumor formation in hepatocellular, cervical, lung, gastric and breast cancer | [7,8] |
| SOX2 | 3q26.33 | Maintains stemness of embryonic cells | High expression with poor prognosis in breast, oral, lung, ovarian and prostate cancer | [9] | |
| SOX3 | Xq27.1 | Development of pituitary and CNS | Promotes malignant behaviour of glioblastoma cells Induces EMT in osteosarcoma | [10-12] | |
| Group B2 | SOX14 | 3q22.3 | Neuronal development | High expression associated with increased growth and metastasis in cervical cancer | [13,14] |
| SOX21 | 13q32.1 | Neural stem cell differentiation | Induces apoptosis of GBM and glioma | [15,16] | |
| Group C | SOX4 | 6q22.3 | Development of heart, reproductive system and CNS | Upregulated in breast, lung and cervical cancer Induces EMT in breast and gastric cancer | [17-20] |
| SOX11 | 2p25.2 | Development of cerebral cortex, kidney, lung and spinal cord | Elevated expression is associated with B-lineage malignancies | [21,22] | |
| SOX12 | 20p13 | Differentiation of lymphocytes | Associated with progression and poor prognosis in breast cancer Promotes growth of multiple myeloma cells | [23-25] | |
| Group D | SOX5 | 12p12.1 | Chondrogenesis and neural cell development | Promotes invasion and metastasis in gastric cancer Contributes to EMT and metastasis in prostate cancer | [26-28] |
| SOX6 | 11p15.2 | Differentiation of erythroid cells Regulates adipogenesis | Inhibits proliferation, migration, tumor growth and metastasis in pancreatic cancer Low expression associated with poor prognosis in lung adenocarcinoma | [29-32] | |
| SOX13 | 1q32.1 | Brain development Cartilage development | Promotes metastasis in colorectal cancer Promotes breast cancer cell growth and glycolysis | [33-35] | |
| Group E | SOX8 | 16p13.3 | Maintenance of gonadal function Testis determination | Promotes tumor growth and predicts poor prognosis in Tongue SCC Associated with tumor invasion in colorectal cancer | [36-38] |
| SOX9 | 17q24.3 | Development of pancreas and intestine Cartilage and lung development | Master regulator of breast cancer cell survival and metastasis Promotes pancreatic cancer progression by regulating EMT | [39-42] | |
| SOX10 | 22q13.1 | Differentiation of neural crest cells | Prognostic marker for human nasopharyngeal carcinoma Prmotes proliferation ability of melanoma cells | [43-45] | |
| Group F | SOX7 | 8p23.1 | Heart development | Tumor suppressor in lung cancer and glioblastoma | [46-48] |
| SOX17 | 8q11.23 | Hematopoetic development from Stem cells | Immunomarkers for ovarian and endometrial cancer Inhibits metastasis in endometrial cancer | [49-51] | |
| SOX18 | 20q13.33 | Development of blood and lymphatic vessels | Promotes metastasis in gastric cancer | [52,53] | |
| Group G | SOX15 | 17p13.1 | Population of myogenic progenitor cells and regeneration | inhibit the proliferation, migration and invasion and induce cells apoptosis in colorectal cancer | [54] |
| Group H | SOX30 | 5q33.3 | Development of testis and male fertility | Prognostic marker for ovarian cancer Inhibits migration and invasion in lung cancer | [55-57] |
CNS: central nervous system, EMT: Epithelial-to-mesenchymal transition, SCC: Squamous cell carcinoma, GBM: Glioblastoma multiforme
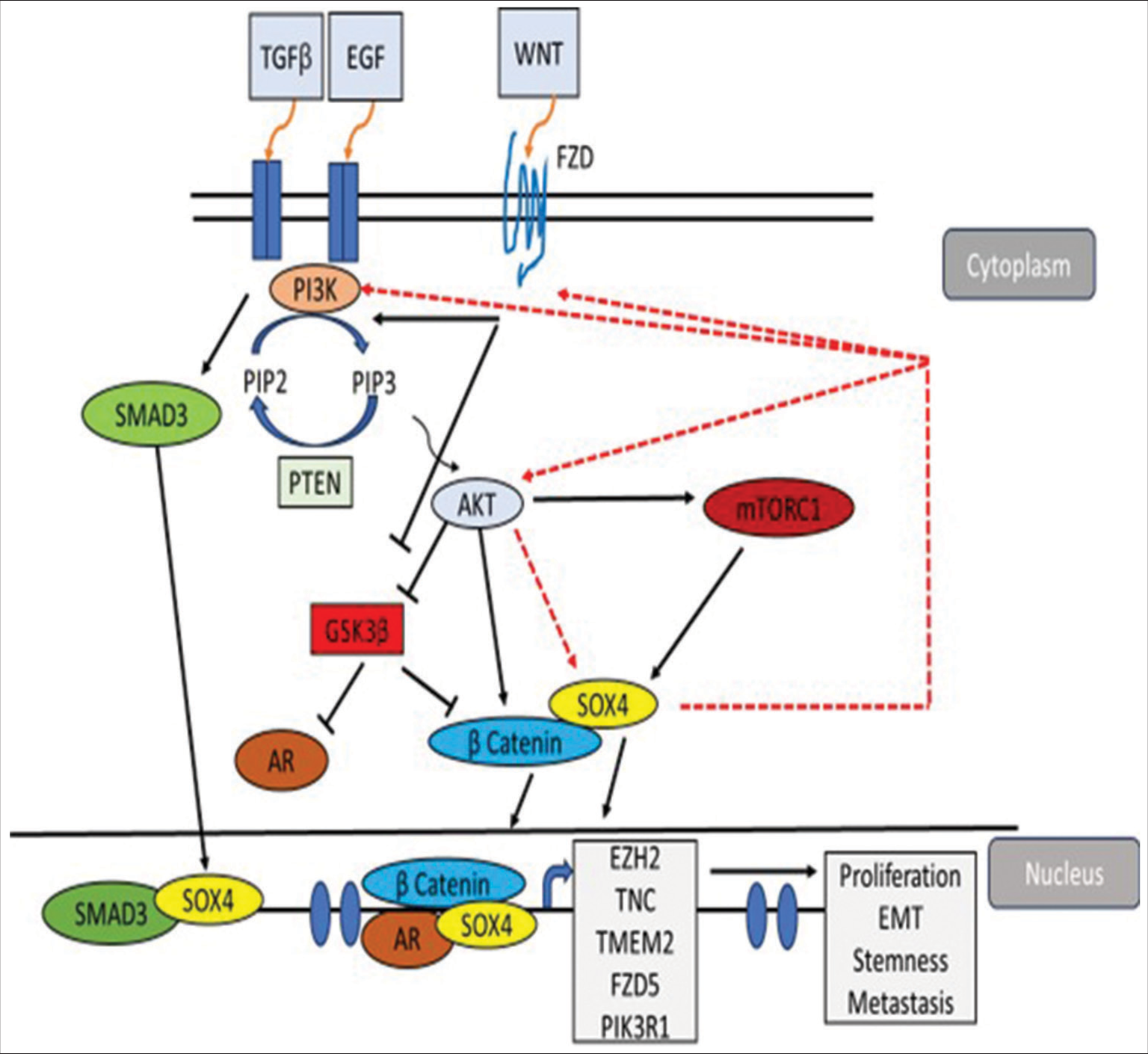
- Schematic diagram illustrating how SOX4 interacts with PI3K-AKT, Wnt/β-catenin, SMAD3, and AR signaling pathways, and how the PI3K-AKT-mTOR pathway regulates the expression of SOX4 in cancer progression. A potential positive feedback loop is indicated by the red dashed line. Transforming growth factor-β and Wnt activate PI3K/AKT to induce and stabilize SOX4 protein. SOX4 recruits β-catenin and interacts with SMAD3 at promoters of genes critical for proliferation, stemness, epithelial-to-mesenchymal transition, and metastasis.
The interaction between SOX4 targeting microRNAs (miRNAs) and long non-coding RNAs (lncRNA) and the consequent epigenetic regulation of tumorigenesis has gained attention in recent years and is employed in cancer therapeutics.
SOX4 AS AN ESSENTIAL DEVELOPMENTAL TRANSCRIPTION FACTOR
The crucial transcription factor SOX4 controls progenitor development, differentiation, stemness, and a variety of pathways contributing to development.[17,60,61] It has been observed that the knockout of SOX4 in vivo led to embryonic lethality due to cardiac failure.[62] Moreover, in SOX4 deficient embryos, the development of B lymphocytes is hindered as there is a halt at the pro-B cell stage.[62] In addition to being expressed in stem cells, SOX4 regulates stem cell activation[63] and also contributes to stem cell maintenance, possibly by activating the expression of SOX2.[20,64,65] The cytokine-induced granulocyte maturation in the 32Dcl3 murine myeloid cell line was significantly suppressed by SOX4 overexpression, indicating a differentiation block.[66] Furthermore, the establishment of a fully mature phenotype is inhibited by prolonged SOX4 expression in the cells of the central nervous system, indicating that premature terminal differentiation may be prevented by SOX4.[67] Around the time when adult neural stem cells (NSCs) commit to a neuron, SOX4 expression is initiated in the adult mouse hippocampus and is restored in immature neurons. SOX4 expression also facilitates and is required for the generation of neurons from adult NSCs in vitro.[68] SOX4 protects tyrosine hydroxylase-expressing cells by acting as a pro-survival factor throughout the development of the spinal cord.[69] Therefore, it can be concluded that SOX4 is a crucial transcription factor for development, which promotes stemness, early differentiation, growth, and survival of transit-amplifying progenitors and regulates multiple pathways corresponding to normal development.
ASSOCIATION OF SOX4 WITH VARIOUS MALIGNANCIES
SOX4 transcription factor is involved in tumorigenesis and regulates various aspects of cancer development. SOX4 was found to be 1 of 64 genes which are upregulated as a “general cancer signature” in transcriptional profiles of human cancers,[70] highlighting the function of SOX4 in malignancies.
Multiple studies have shown that aberrant expression of SOX4 is linked to the progression of various cancers. SOX4 protein is upregulated in cancers of the prostate,[71] endometrial,[72] and breast[73] and exerts an oncogenic function, whereas a reduced expression of SOX4 is detected in cutaneous melanoma,[74] primary gallbladder carcinoma (PGC),[75] and colon cancer,[76] where SOX4 plays the role of cancer suppressor gene.
Several facets of cancer development and progression are promoted by elevated expression of SOX4 and it also regulates expression of many downstream genes and signaling pathways. SOX4 contributes to the malignant phenotypes of cancer cells by regulating processes such as epithelial-to-mesenchymal transition (EMT) and cell death through apoptosis. Several evidences have shown that SOX4 knockdown resulted in apoptosis in adenoid cystic carcinoma (ACC),[77] melanoma cells,[78] and lung cancer[79] and contrary to that SOX4 overexpression promoted cell cycle and apoptosis in colon cancer[76] and hepatocellular carcinoma (HCC).[80] SOX4 overexpression increased cell invasion and metastasis by inducing EMT in renal cell carcinoma[81] and gastric cancer (GC)[20], whereas SOX4 knockdown increased EMT in bladder cancer.[82]
SOX4 expression is a prognostic factor in determining cancer patient outcomes. Higher expression of SOX4 is seen to be associated with better prognosis in HCC,[80] medulloblastoma,[83] and bladder cancer[84] patients but shorter survival in patients of prostate cancer,[85] GC,[86] and colon cancer.[87]
SOX4 is known to serve as a specific predictor of chemotherapy response in cancer. One of the important findings suggests that ectopic expression of SOX4 increases the susceptibility of breast cancer cells to paclitaxel[88], whereas the cell death induced by cisplatin reduced sharply with SOX4 overexpression in cervical cancer[89] and oral squamous cell carcinoma (OSCC).[90]
SOX4 in Adenoid Cystic Carcinoma (ACC)
Microarray analysis of RNA gene expression profiling in ACC shows that SOX4 is one of the most upregulated genes. SOX4 protein was upregulated in ACC patients, as revealed by immunohistochemistry of 20 normal tissues and 28 primary cancers. Silencing SOX4 protein reduced cell viability with an increase in apoptosis, suggesting that SOX4 contributes to tumorigenesis by promoting ACC cell survival. Cell morphology, DNA fragmentation, and flow cytometry were used to confirm apoptosis. Overall, these findings suggested that SOX4 is overexpressed in ACC patients and promotes the survival of ACC cells by contributing to the malignant phenotypes.[77]
SOX4 in Bladder Cancer
Whole-genome expression analysis of bladder tumor tissue samples and normal tissues was used to determine the involvement of SOX4 in bladder cancer disease. SOX4 expression was five-fold higher in bladder tumor tissues as compared to normal tissues. Tissue microarray analysis with clinical annotation was used to evaluate SOX4 protein expression in bladder cancers, and a relation between SOX4 expression and improved patient survival was observed. In the HU609 bladder cancer cell line, overexpression of SOX4 significantly reduced cellular viability and increased apoptosis.[84] In another study, SOX4 expression was investigated in bladder cancer and normal bladder tissues. In comparison to normal tissue, expression of the SOX4 gene was 2.2-fold upregulated in bladder tumor tissues. A significant variation was also observed in immunostaining between normal bladder and bladder tumor tissues. SOX4 was strongly expressed in tumor tissues. These results suggested that SOX4 might be involved in bladder cancer tumorigenesis.[91]
SOX4 in Breast Cancer
Various studies have shown a correlation of SOX4 transcription factor with increased tumor size, facilitating migration and invasion, leading to metastasis and poor overall survival (OS) in breast cancer patients.[92] Another study revealed that there was an aberrant overexpression of SOX4 which correlated with the triple-negative breast cancer (TNBC) (ER−/PR−/HER−) subtype in human breast cancer specimens.[73]
Transforming growth factor-β (TGF-β) directly targets SOX4. SOX4 induces TGF-β and is essential for TGF-β-induced EMT. Ectopic expression of SOX4 n mammary epithelial cells upregulated the expression of inducers of EMT and TGF-β pathway. It has also been demonstrated that conditional activation of SOX4 could not induce complete EMT. Silencing of SOX4 using short hairpin RNA (shRNA) approach, delayed messenger RNA (mRNA), and protein expression of TGF-β induced mesenchymal markers. Collectively, these findings indicate that in human primary epithelial cells, TGF-β-mediated increased expression of SOX4 was essential to induce a mesenchymal phenotype during EMT.[58]
mRNA and protein expression of SOX4 was evaluated in breast cancer tissues and adjacent non-cancer tissues and its relation with clinical features of breast cancer patients was studied. Compared to adjacent normal mammary tissues, it was seen that mRNA and protein expression of SOX4 was upregulated in breast cancer tissues and correlated positively with clinical stages, tumor-node-metastasis (TNM) classification, hormone receptor status, and histological grades of breast cancer patients. Overexpression of SOX4 protein was an unfavorable prognostic factor and biomarker, regardless of tumor size, clinical stage, lymph node, or distant metastasis in breast cancer patients.[93]
Our group has also shown SOX4 protein expression in different breast cancer cell lines (unpublished data). We have observed that gene silencing of SOX4 in BT-474 breast cancer cells resulted in increased apoptosis and promoted autophagy (unpublished data).
SOX4 in Cervical Cancer
According to recent reports, SOX4 is highly expressed in cervical cancer cells and tissues. In CaSki cervical cancer cells, overexpression of SOX4 promotes the formation of tumor clones, increases cellular proliferation, and accelerates the cell cycle. On the other hand, silencing of SOX4 using the shRNA approach in CaSki cells inhibited cellular proliferation and decreased the progression of the cell cycle, suggesting the contribution of SOX4 in tumorigenesis. In addition, overexpression of SOX4 by gene transfer decreased the sensitivity of CaSki cells toward the chemotherapeutic drug cisplatin, whereas silencing SOX4 expression using an RNA interference approach increased the sensitivity of CaSki cells in response to cisplatin. Moreover, ABCG2, which is a multiple drug resistant gene, was upregulated by overexpression of SOX4 whereas SOX4 downregulation inhibited expression of ABCG2. All these findings collectively show that SOX4 modulates the proliferation of cancer cells by cell cycle regulation and inhibition of cancer cell sensitivity toward therapeutic drugs through upregulating ABCG2.[89] Therefore, it could be concluded that SOX4 might serve as a focus molecule for cervical cancer treatment.
SOX4 in Chondrosarcoma
It has been shown that SOX4 is overexpressed in 30.4% of interpretable chondrosarcoma patients as compared to 6.9% of interpretable chondroma cases. SOX4 overexpression significantly correlated with both histological grade and tumor recurrence in chondrosarcoma for patients with low histological grade chondrosarcoma; SOX4 was an unfavorable independent prognostic factor. Downregulation of SOX4 in vitro suppressed the proliferation migration and invasion capability of SW1353 cells, suggesting the oncogenic function of SOX4 in chondrosarcoma. Clinically, miR-30a and SOX4 expression are negatively correlated in chondrosarcoma cases. In conclusion, SOX4 serves as an unfavorable prognostic factor for low histological grade chondrosarcoma patients.[94]
SOX4 in Esophageal Cancer
Activation of SOX4 is associated with downregulation of miRNA in esophageal carcinoma. Esophageal tumor samples collected from patients who underwent primary surgical resection showed an elevated expression of SOX4 as compared to controls. SOX4 expression was upregulated, whereas expression of miR-129-2 was low in primary esophageal tumors. This finding revealed that dysregulation of miR-129-2 causes aberrant SOX4 expression, and miRNA restoration suppresses the oncogenic target of SOX4 in esophageal cancer.[95]
One of the genes that miR-133a directly targets is SOX4. miR-133a expression was seen to be lowered in cells and tissues of esophageal squamous cell carcinoma (ESCC). Expression of SOX4 correlated inversely with ESCC tissue samples. Furthermore, ablation of SOX4 led to a reversal in the increased migration and invasion mediated by anti-miR-133 in ESCC.[96]
Around 90% of cancer-related deaths occur due to metastasis of tumors. According to a recent report, to ablate miR-31, SOX4 cooperates with EZH2 and HDAC3 to activate and stabilize a co-repressor complex, hence promoting the growth and invasion of esophageal tumor cells. On binding of this co-repressor complex to the miR-31 promoter, epigenetic repression of miR-31 occurs. Clinical samples also show increased expression of SOX4, EZH2, and HDAC3 in metastatic ESCC tissues in comparison to adjacent normal tissues.[97]
SOX4 in Gallbladder Cancer
Recently, the role of SOX4 and its prognostic significance in PGC was investigated in 136 PGC tissues. SOX4 expression was observed in 75% of PGC tissues but not in normal gallbladder epithelium. In addition, SOX4 overexpression was significantly associated with low pathologic T stage, early clinical stage, and histologic grade. Tissues of PGC with positive nodal metastasis had lower levels of SOX4 immunostaining than tissues without metastasis. Furthermore, Kaplan–Meier curves revealed that SOX4 overexpression was substantially associated with better overall and disease-free survival (DFS). In PGC patients, SOX4 overexpression served as a sole predictor of improved overall DFS and correlated significantly with positive clinicopathologic characteristics.[75]
SOX4 in Gastric Cancer (GC)
In GC tissues, the relation of SOX4 expression with patient survival and other clinicopathological features was examined. SOX4 overexpression correlated significantly with stage, nodal status, vascular invasion, distant metastasis, and poorer DFS rate. Overexpression of SOX4 serves as a prognostic biomarker for GC and can be exploited to determine the outcome of GC patients.[86]
Another study shows that SOX4 promotes GC progression by regulating EMT and acquiring stemness through the activation of transcription factors and the Wnt signaling pathway. TGF-β signaling targets SOX4 and induces EMT and stem cell features of GC cells, revealing that the TGF-β/SOX4 axis regulates the malignant behavior of GC cells.[20]
Subgroup analyses revealed that overexpression of SOX4 was a poor predictor of OS for GC patients and recurrence-free survival for colorectal cancer (CRC) patients. In conclusion, it can be determined that SOX4 may be a beneficial prognostic biomarker for GC in humans.[98]
SOX4 in Head and Neck Cancer
The impact of SOX4 on oncogenic behavior and responsiveness to chemotherapy and radiation therapy in cells of head and neck squamous cell carcinoma (HNSCC) was assessed. SOX4 ablation decreased cellular proliferation and induced apoptosis through activation of caspase-3, caspase-7, and poly-ADP ribose polymerase and inhibiting X-linked inhibitor of apoptosis protein in HNSCC cells. Moreover, it promoted apoptosis induced by radiation/cisplatin and downregulated migration and invasion of HNSCC cells. SOX4 protein expression was also significantly elevated in OSCC tissues as compared to adjacent normal mucosa. It was concluded that SOX4 may contribute to malignant phenotypes of HNSCC cells by enhancing their survival.[90]
SOX4 in Hepatocellular Carcinoma (HCC)
The gene regulation and function of the SOX4 were investigated in metastatic HCC. RNAi-mediated silencing of SOX4 reduced migration and invasion of tumor cells, suggesting that SOX4 regulates tumor metastasis and also in vivo tumorigenesis. Silencing of two target genes of SOX4 – Semaphorin 3C and Neuropilin 1, reduced migration ability in HCC cells indicating SOX4 also exerts some of its effects through regulating downstream target genes.[99]
SOX4 also contributed to carcinogenesis by inhibiting apoptosis mediated by p53, and its overexpression may function as a beneficial prognostic marker for better management of HCC.[80]
SOX4 in Leukemia
It was discovered that primary adult T-cell leukemia (ATL) cells consistently expressed SOX4. Knockdown of SOX4 expression suppressed the growth of ATL leukemia cells.[100]
C/EBPα directly targets SOX4, and its expression inversely correlates with C/EBPa activity. Leukemic cells that self-renewal were abrogated by downregulation of SOX4, and their differentiation was restored. This data demonstrates the development of leukemia with a distinct phenotype by SOX4 expression mediated by C/EBPa inactivation.[101] Poor outcome was predicted by high levels of SOX4 expression in acute lymphoblastic leukemia (ALL) cells at the time of diagnosis in a pediatric clinical trial (COG P9906). These studies justify that SOX4 centrally mediates oncogenic signaling in ALL.[102]
SOX4 in Lung Cancer
SOX4 gene mutation was investigated in non-small-cell lung cancer (NSCLC) tissues[103], and it was not associated with the histopathology of tumors but related to pathological stages. The rate of mutation increased gradually with a positive relation in advanced-stage NSCLC. The results revealed that mutations in the SOX4 gene contribute to lung carcinogenesis.[103]
mRNA expression levels of SOX4 were compared in lung cancer cell lines.[104] In cells with gene amplification, there was an overexpression of the SOX4 gene by a factor of 10. The fraction of primary lung tumors and lung cancer cell lines expressed SOX4 more strongly. Results showed that overexpression of SOX4 is due to the amplification of genes and provided the basis for the malignant role of SOX4 in lung cancer.[104]
SOX4 gene is directly targeted by miR-132. Biological functions and molecular mechanisms of miR-132 were evaluated in human lung cancer cells. Expression levels of miR-132 were downregulated in lung cancer cell lines A549, YTMLC-9, and H460. Furthermore, miR-132 led to significant downregulation in in vitro migration and invasion ability of lung cancer cells. Overexpression of miR-132 also inhibited the in vivo growth of the tumor. Reintroducing SOX4 reversed miR-132’s anti-invasion function.[105]
SOX4 expression analysis and its relationship with clinicopathological characteristics were evaluated in NSCLC.[106] As compared to normal tissues, mRNA and protein expression of SOX4 was higher in NSCLC tissues and correlated positively with the status of degree of differentiation, clinical stage, and TNM classification. Moreover, elevated expression of SOX4 was related to poor OS in NSCLC patients, indicating a crucial role of SOX4 in the advancement and prognosis of NSCLC.[106]
SOX4 in Melanoma
SOX4 expression was evaluated in 180 melanocytic lesions using a tissue microarray. Expression of SOX4 was lower in metastatic melanoma, in comparison to primary melanoma and dysplastic nevi and significantly related with a poorer disease-specific survival of melanoma patients. Knockdown of SOX4 increased migration and invasion abilities and also upregulated stress fiber formation of melanoma cells. Results suggested that SOX4 regulates the invasion and migration of melanoma cells and could be used as a prognosis indicator and possible treatment target for human melanoma.[74]
SOX4 in Pancreatic Cancer
According to recent reports, SOX4 regulates several EMT master regulators, such as Twist, Zeb, and Snail, and is essential for EMT both in vivo and in vitro. In addition, SOX4 promotes EMT and metastasis by reprogramming the cancer epigenome by inducing the transcription of Ezh2 (histone methyltransferase). In pancreatic cancer, Exh2 and miR-335 regulate the expression of SOX4 epigenetically. The prognosis for DFS was worse for SOX4-positive patients, and the prognosis for OS was similarly significantly lower for Ezh2-positive individuals. It has been discovered that the SOX4/Ezh2 axis influences prognosis in pancreatic cancer patients.[107]
SOX4 in Prostate Cancer
Expression of SOX4 correlated highly with Gleason score at gene and protein levels in prostate tumor samples. Silencing of SOX4 induced apoptosis of prostate cancer cells, indicating that SOX4 could serve as a therapeutic target for prostate cancer.[71]
Expression of SOX4 was higher in castration-resistant prostate cancer (CRPC) tumors as compared to hormone-dependent prostate cancer. The androgen-depleted environment promotes prostate cancer cell proliferation mediated by 17b-estradiol. In addition, SOX4 gene silencing attenuates the proliferation migration and invasion ability of prostate cancer cells. Thus, it could be concluded that identification and prevention of the onset of CRPC may be aided by SOX4 overexpression.[108]
EPIGENETIC REGULATION OF SOX4
Various epigenetic mechanisms, such as DNA or histone methylation, can lead to altered SOX4 expression. Although SOX4 is overexpressed in a number of malignancies, SOX4 gene amplification is reported in only a few cancer types.[102,104] Other regulatory mechanisms, such as lncRNA and miRNA-mediated processes, lead to elevated expression of SOX4 in cancer.
miRNA mediation regulation of SOX4 in human cancers
In human malignancies, miRNAs are potent regulators of gene expression. These can function as oncogenes (oncomiRs) or tumor suppressors, depending on the particular malignancy and miRNA involved. While most research has focused on individual targets of miRNAs, they actually influence multiple genes at once, often in connected pathways. This complex web of interactions makes miRNAs important players in cancer biology.[59]
It has been discovered that a large number of miRNAs directly bind to the 3’untranslated region (UTR) of SOX4 to target it. This regulates several critical processes in cancer cells, including tumor growth, proliferation, invasion, migration, and EMT. A recent study has shown that miR-355 suppresses invasion and metastasis, targeting the SOX4 in breast cancer.[109] Another study identified miR-191-5p as an oncogene in various human cancers.[110-112] miR-187 targets SOX4, and its inhibition was linked to metastasis and dismal prognosis in CRC.[113] Overexpression of miR-138 in GC inhibited tumor growth in vivo and suppressed in vitro cell proliferation, colony formation, migration, and invasion.[114] In HCC, epigenetic inhibition of miR-130a-3p resulted in increased SOX4 expression.[115] MiR-133a directly targets 3’UTR in esophageal cancer and its downregulation aids in the development of ESCC.[96] In prostate cancer, also miR-30a directly targets SOX4 3’UTR, and reduced miR-30a levels are linked to SOX4 overexpression, which also inhibits PC cell growth and the EMT process.[116] In osteosarcoma, miR-25-3p reduced cell proliferation, colony formation, migration, and invasion in vitro and slowed tumor growth in vivo through targeting SOX4 3’UTR.[117]
lncRNAs-mediated regulation of SOX4 in human cancers
During chromatin remodeling, lncRNAs and RNA-binding proteins work together to regulate a range of dynamic and epigenetic processes. lncRNAs work at the transcriptional, posttranscriptional, and translational levels.[118] They can also influence the activity of mRNA-targeting miRNAs by functioning as competing endogenous RNAs (ceRNAs).[119] In a recent study, lncRNA-UCA1 acted as ceRNA for miR-204, which prevented it from attaching to the SOX4 3’UTR and, elevated SOX4 expression, and accelerated the growth of esophageal cancer cells.[120] Similarly, androgen receptor negatively induced lncRNA (ARNILA) served as a ceRNA for miR 204 in TNBC cells, increasing SOX4 expression, inducing EMT, and facilitating invasion and metastasis.[121] It was shown that in HCC, STAT3 signaling induces the production of HOXD-AS1 lncRNA, which inhibits the repression of SOX4 mediated by miR-130a-3p by sequestering it and leads to increased metastasis.[115] In addition, it has been shown that lncRNA-PAGBC stimulates the Akt/mammalian target of the rapamycin pathway and facilitates the metastasis of gallbladder tumors by downregulating miR-133b and miR 511, which, in turn, results in the upregulation of SOX4 and phosphoinositide 3-kinase regulatory subunit 3, respectively.[122] LncRNA ABHD11-AS1 has been demonstrated to stimulate CRC formation by facilitating SOX4 expression by acting as a ceRNA for miR-133a.[122]
Numerous studies have found miRNAs that target SOX4 and have drawn attention to the effects of changing miRNA expression on the regulation and/or suppression of cancer growth. Indeed, across a variety of human cancers, over 25 miRNAs that target SOX4 have been discovered, which is uncommon in other biological systems. SOX4 might be vulnerable to miRNA regulation because it has an unusually long 3’UTR over 4000 nucleotides long. This extended UTR provides more space for many different miRNAs to attach and potentially control SOX4 activity. LncRNAs either function as ceRNAs to sequester miRNAs that target SOX4, or they can increase SOX4 expression. This intricate network of regulatory networks emphasizes the critical importance of SOX4 and the level of regulation needed to maintain the optimal balance of this master regulator to avoid tumorigenesis. Even though SOX4’s function in tumorigenesis and epigenetic regulation has been extensively studied, there are still many unanswered concerns about this protein’s epigenetic regulation and possible therapeutic applications.
SOX4 AS A POTENTIAL PROGNOSTIC BIOMARKER FOR HUMAN CANCERS
SOX4 plays a complex role in cancer, with its expression differing depending on the cancer type. In many cancers, such as those of the bladder,[84] prostate,[71] endometrium,[72] and liver,[80] SOX4 expression is elevated. However, contrary to that expression of SOX4 is lower in melanoma[74] and gallbladder cancer.[75] Numerous investigations have examined the relationship between nuclear SOX4 expression and clinicopathologic markers as well as the implications of nuclear SOX4 to predict prognosis of cancer patients.
Nuclear SOX4 expression was elevated in colon cancer and correlated with stage, distant metastasis, and depth of invasion. In addition, it was linked to a worse DFS rate. Compared to patients with low expression levels, those with high nuclear SOX4 expression levels had a 5-year DFS rate of 44.8%. Nevertheless, in multivariate analysis, when other known prognostic factors such as nodal status, stage, and degree of differentiation were included, the correlation between overexpression of nuclear SOX4 and survival was not statistically significant (P = 0.425), whereas distant metastasis (Hazard Ratio [HR] = 0.052, 95% confidence interval [CI] = 0.024–0.111, P ≤ 0.001] was prognostically independent.[87]
In GC, the following parameters showed significant correlations with nuclear SOX4 overexpression: Depth of invasion, nodal status, distant metastasis, stage, and vascular invasion. SOX4 overexpression was linked to a considerably lower disease-free survival. Patients with higher nuclear SOX4 expression levels had a 5-year DFS rate of 45.6%, while those with lower SOX4 expression levels had a higher DFS rate of 66.5%. Nevertheless, no statistically significant correlation between nuclear SOX4 overexpression and survival was found in multivariate analysis. Prognostic factors in multivariate analysis included nodal status (HR = 3.901, 95% CI = 1.589–9.580, P = 0.003), distant metastasis (HR = 15.453, 95% CI = 6.419–37.114, P = 0.001), depth of invasion (HR = 2.091, 95% CI = 1.073–4.077, P = 0.030), and vascular invasion (HR = 1.849, 95% CI = 1.058–3.229, P = 0.031).[86]
Patients with strong or moderate nuclear SOX4 immunopositivity (overexpression) were found to have a better prognosis than those with weak or negative nuclear SOX4 expression in cases of HCC, whereas clinicopathological parameters and SOX4 expression did not correlate.[80]
In laryngeal squamous cell carcinoma (LSCC), pathological differentiation, lymphatic invasion, and pathological TNM were all associated with SOX4 expression. Multivariate analysis revealed that lymphatic invasion and positive SOX4 expression were independent predictors of the OS time of LSCC patients following surgery.[123]
PROGNOSTIC MODELS BASED ON SOX4-ASSOCIATED GENES
It is commonly known that a combination of genetic, environmental, dietary, and physical factors can shape a tumor. The development of reliable models that can predict the prognosis is critically important. As it is widely known, aberrant DNA damage, EMT, M6A methylation, hypoxia, energy metabolism, and ferroptosis are important processes frequently involved in tumor development and progression.[124-129] Thus, based on the SOX4-associated genes, six multi-gene prognostic models have been developed to predict the prognosis of liver HCC. These models include genes linked to DNA damage repair, EMT, M6A methylation, hypoxia, energy metabolism, and ferroptosis. The estimated 1-, 3-, and 5-year survival probability was calculated with the help of nomograms. The area under the receiver operating characteristic curves (AUCs) for the 1, 3, and 5-year survival rates also showed that all models displayed good predictive discrimination.[130]
To establish the prognostic model based on the SOX4-associated genes related to energy metabolism, ferroptosis, hypoxia, EMT, DNA damage repair, and hypoxia, a thorough literature search was carried out. Cluster heatmaps were then used to visualize the correlations between these genes and SOX4 expression in a variety of tumor types. To identify the genes with non-zero coefficients, the least absolute shrinkage, and selection operator regression approach was applied. Independent risk factors for OS, two-sided P < 0.05, were identified using a multivariate logistic regression, and the risk was then predicted using a nomogram model based on associated genes. The AUC of the receiver operating characteristic curve was used to measure the nomogram’s prediction accuracy. The Kaplan–Meier survival analysis was utilized to determine the OS of LHIC patients.[131] Risk factors of SOX-4 associated genes based on multivariate analysis are shown in Tables 2-7.
| Variable | Model | ||
|---|---|---|---|
| DNA damage- related genes | β-coefficient | Odds Ratio (95% CI) |
P-value |
| RFC3 | −0.574 | 0.563 (0.415, 0.763) | <0.001 |
| RAD54B | 0.876 | 2.401 (1.006, 5.728) | 0.048 |
| MUTYH | 0.407 | 1.503 (1.010, 2.236) | 0.044 |
| MGMT | −0.418 | 0.658 (0.509, 0.851) | 0.001 |
| HAP1 | 0.582 | 1.789 (1.232, 2.598) | 0.002 |
| UVSSA | −0.456 | 0.634 (0.411, 0.978) | 0.039 |
CI: Confidence interval
| Variable | Model | ||
|---|---|---|---|
| EMT-related genes | β-coefficient | Odds Ratio (95% CI) |
P-value |
| ACTA2 | −0.235 | 0.791 (0.671, 0.933) | 0.005 |
| PHLDA2 | 0.186 | 1.204 (1.070, 1.355) | 0.002 |
| SGCB | 0.262 | 1.299 (1.118, 1.509) | <0.001 |
| NKX3-2 | 0.403 | 1.497 (1.059, 2.116) | 0.022 |
EMT: Epithelial-to-mesenchymal transition, CI: Confidence interval
| Variable | Model | ||
|---|---|---|---|
| M6A methylation- related genes | β-coefficient | Odds Ratio (95% CI) |
P-value |
| ZC3H13 | −0.456 | 0.634 (0.476, 0.843) | 0.002 |
| YTHDF2 | 0.669 | 1.951 (1.207, 3.154) | 0.006 |
CI: Confidence interval.
| Variable | Model | ||
|---|---|---|---|
| Hypoxia- related genes | β-coefficient | Odds Ratio (95% CI) |
P-value |
| CUL2 | 0.405 | 1.500 (1.019, 2.208) | 0.04 |
| EPO | 0.148 | 1.160 (1.059, 1.270) | 0.001 |
| UBB | −0.443 | 0.642 (0.450, 0.916) | 0.014 |
CI: Confidence interval
| Variable | Model | ||
|---|---|---|---|
| Energy- metabolism- related genes | β-coefficient | Odds Ratio (95% CI) | P-value |
| GGT3P | 2.596 | 13.410 (2.186, 82.250) | 0.005 |
| LDHA | 0.305 | 1.356 (1.015, 1.813) | 0.04 |
| NQO2 | 0.236 | 1.266 (1.031, 1.556) | 0.025 |
CI: Confidence interval
| Variable | Model | ||
|---|---|---|---|
| Ferroptosis- related genes | β-coefficient | Odds Ratio (95% CI) |
P-value |
| SAT1 | −0.283 | 1.115 (0.576, 0.986) | 0.039 |
| SLC7A11 | 0.192 | 1.212 (1.029, 1.426) | 0.021 |
| CISD1 | 0.354 | 1.425 (1.020, 1.992) | 0.078 |
CI: Confidence interval
The STRING database (version 11, Swiss Institute of Bioinformatics, Lausanne, Switzerland) provided a number of proteins that frequently linked with SOX4, which were obtained to explore SOX4 gene-related pathways that may be involved in different kinds of malignancies [Figure 2]. TP53, POU3F3, TCF7, TCF7L1, TCF7L2, CTNNB1, and PTEN were among the predicted interacting proteins with high confidence. The investigation of KEGG pathways demonstrated that specific functional protein partners belonged to pathways associated with the WNT signaling network, the cell cycle signaling route, the p53 signaling pathway, the Hippo signaling pathway, and multiple other pathways linked to cancer.[131]
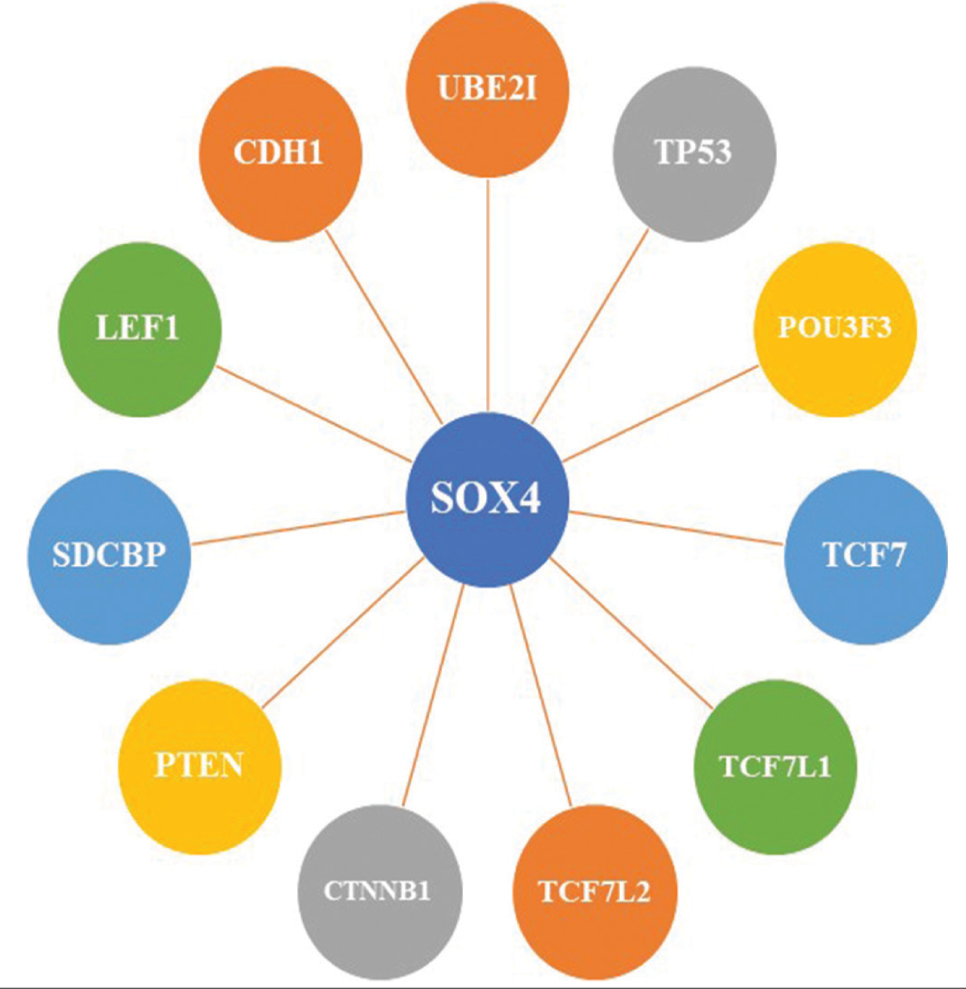
- Functional protein partners of SOX4.
FUTURE PROSPECTIVE
Recently, many studies have put forth evidence that SOX4 plays a significant role in cancer development and progression and relates to poor clinical outcomes in cancer patients. Since SOX4 has been shown to promote the growth and spread of many human cancers by mediating cell survival, stemness, angiogenesis, movement, and metastasis, it holds promise as a target for new cancer therapies. SOX4 is a protein with potential applications in cancer treatment. However, it is challenging to design drugs that target SOX4 effectively for two reasons. First, SOX4 is relatively unstructured outside its HMG DNA binding domain (DBD), making it difficult to create crystals for studying its properties. Second, SOX4 DBD is very similar to other proteins in the SOX family. This similarity makes it difficult to develop drugs that target only SOX4 and no other SOX proteins. Specificity and selectivity remain key issues; therefore, further research is needed to address these concerns.
Numerous studies have identified miRNAs that target SOX4 and have brought attention to the effects of changing miRNA expression on the regulation and suppression of cancer growth. There is a wealth of preclinical data that show miRNA-based therapeutics that are being used in vivo in a variety of disease models. In regard to cancer, therapeutics miRNA mimics have entered into clinical trials and shown plausible results. Research shows promise for turning epigenetic therapies into cancer treatments, but more work is needed to develop therapies that are precise and have minimal side effects. Specifically, creating small molecule inhibitors targeting SOX4 and ensuring a smooth transition from preclinical data to clinical trials are important next steps.
By controlling the expression of multiple other genes and the downstream signaling pathways these genes activate, SOX4 mediates development and carcinogenesis and may be a useful target for cancer treatment. Even though it has been demonstrated that numerous SOX proteins are critical for cell survival, tumor cell growth and proliferation, and a multitude of other tumor features, there are still a number of concerns to be addressed, such as the difficulty of therapeutically blocking transcription factors.
Subsequent investigations will yield a further understanding of the interactome and gene regulatory networks of the SOX4 protein in relation to cancer. Without a doubt, these findings will contribute to the creation of new treatment approaches for this extremely diverse disease with fewer available treatments. Few promising findings point to the potential of SOX4 in cancer management but warrant further research, which may be valuable for the management of the overall burden of cancer.
CONCLUSION
By transcriptional activation and altering downstream genes epigenetically, SOX4 facilitates various facets of cancer development and progression, giving malignant cells an edge in proliferation and survival. The idea that SOX4 is an oncogene that governs angiogenesis, migration, EMT, metastasis, stemness, and cancer cell survival is well supported by the literature. Thus, targeting SOX4-mediated pathways may provide therapeutic opportunities for various highly aggressive human cancers.
Therefore, an improved comprehension of the roles of SOX4 in various cancers and its underlying mechanisms may offer newer insights into tumor development and progression and facilitate in discovery of potent anti-cancer therapies.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient consent was not required as there are no patients in this study.
Conflicts of interest
Dr. Nirmal Kumar Lohiya is on the editorial board of the journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Financial Support provided by National Institute of Immunology, New Delhi and Mahatma Gandhi University of Medical Sciences and Technology, Jaipur.
References
- Genetic evidence equating SRY and the testis-determining factor. Nature. 1990;348:448-50.
- [CrossRef] [PubMed] [Google Scholar]
- A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240-4.
- [CrossRef] [PubMed] [Google Scholar]
- A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245-50.
- [CrossRef] [PubMed] [Google Scholar]
- SOX family transcription factors involved in diverse cellular events during development. Eur J Cell Biol. 2015;94:547-63.
- [CrossRef] [PubMed] [Google Scholar]
- SRY and OCT4 are required for the acquisition of cancer stem cell-like properties and are potential differentiation therapy targets. Stem Cells. 2015;33:2652-63.
- [CrossRef] [PubMed] [Google Scholar]
- SRY-related transcription factors in head and neck squamous cell carcinomas: In silico based analysis. Curr Issues Mol Biol. 2023;45:9431-49.
- [CrossRef] [PubMed] [Google Scholar]
- SOX1 is a backup gene for brain neurons and glioma stem cell protection and proliferation. Mol Neurobiol. 2021;58:2634-42.
- [CrossRef] [PubMed] [Google Scholar]
- SOX1 acts as a tumor hypnotist rendering nasopharyngeal carcinoma cells refractory to chemotherapy. Cell Death Discov. 2023;9:194.
- [CrossRef] [PubMed] [Google Scholar]
- SOX2 in development and cancer biology. Semin Cancer Biol. 2020;67:74-82.
- [CrossRef] [PubMed] [Google Scholar]
- SOX transcription factors as important regulators of neuronal and glial differentiation during nervous system development and adult neurogenesis. Front Mol Neurosci. 2021;14:654031.
- [CrossRef] [PubMed] [Google Scholar]
- SOX3 can promote the malignant behavior of glioblastoma cells. Cell Oncol (Dordr). 2019;42:41-54.
- [CrossRef] [PubMed] [Google Scholar]
- Sex-determining region Y-box protein 3 induces epithelial-mesenchymal transition in osteosarcoma cells via transcriptional activation of Snail1. J Exp Clin Cancer Res. 2017;36:46.
- [CrossRef] [PubMed] [Google Scholar]
- Expression analysis of SOX14 during retinoic acid induced neural differentiation of embryonal carcinoma cells and assessment of the effect of its ectopic expression on SOXB members in HeLa cells. PLoS One. 2014;9:e91852.
- [CrossRef] [PubMed] [Google Scholar]
- Cervical cancer progression is regulated by SOX transcription factors: Revealing signaling networks and therapeutic strategies. Biomed Pharmacother. 2021;144:112335.
- [CrossRef] [PubMed] [Google Scholar]
- SOX5/6/21 prevent oncogene-driven transformation of brain stem cells. Cancer Res. 2017;77:4985-97.
- [CrossRef] [PubMed] [Google Scholar]
- Dual effects of miR-181b-2-3p/SOX21 interaction on microglia and neural stem cells after gamma irradiation. Cells. 2023;12:649.
- [CrossRef] [PubMed] [Google Scholar]
- Roles of Sox4 in central nervous system development. Brain Res Mol Brain Res. 2000;79:180-91.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 maintains the stemness of cancer cells via transcriptionally enhancing HDAC1 revealed by comparative proteomics study. Cell Biosci. 2021;11:23.
- [CrossRef] [PubMed] [Google Scholar]
- Sox4, EMT programs, and the metastatic progression of breast cancers: Mastering the masters of EMT. Breast Cancer Res. 2013;15:R72.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 contributes to TGF-β-induced epithelial-mesenchymal transition and stem cell characteristics of gastric cancer cells [published correction appears in Genes Dis 2021;8:946-8] Genes Dis. 2017;5:49-61.
- [CrossRef] [PubMed] [Google Scholar]
- Regulatory roles for SOX11 in development, stem cells and cancer. Semin Cancer Biol. 2020;67:3-11.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological features and prognostic value of SOX11 in childhood acute lymphoblastic leukemia. Sci Rep. 2020;10:2043.
- [CrossRef] [PubMed] [Google Scholar]
- Sox12 promotes T reg differentiation in the periphery during colitis. J Exp Med. 2018;215:2509-19.
- [CrossRef] [PubMed] [Google Scholar]
- SOX12 expression is associated with progression and poor prognosis in human breast cancer. Am J Transl Res. 2020;12:8162-74.
- [Google Scholar]
- SOX12 promotes the growth of multiple myeloma cells by enhancing Wnt/b-catenin signaling. Exp Cell Res. 2020;388:111814.
- [CrossRef] [PubMed] [Google Scholar]
- An infant-type hemispheric glioma with SOX5:ALK: A novel fusion. J Natl Compr Canc Netw. 2024;22:e237102.
- [CrossRef] [Google Scholar]
- SOX5 promotes cell invasion and metastasis via activation of Twist-mediated epithelialmesenchymal transition in gastric cancer. Onco Targets Ther. 2019;12:2465-76.
- [CrossRef] [PubMed] [Google Scholar]
- Sox5 contributes to prostate cancer metastasis and is a master regulator of TGF-β-induced epithelial mesenchymal transition through controlling Twist1 expression. Br J Cancer. 2018;118:88-97.
- [CrossRef] [PubMed] [Google Scholar]
- Sox6 cell-autonomously stimulates erythroid cell survival, proliferation, and terminal maturation and is thereby an important enhancer of definitive erythropoiesis during mouse development. Blood. 2006;108:1198-207.
- [CrossRef] [PubMed] [Google Scholar]
- The transcription factor SOX6 contributes to the developmental origins of obesity by promoting adipogenesis. Development. 2016;143:950-61.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of SOX6 as a regulator of pancreatic cancer development. J Cell Mol Med. 2018;22:1864-72.
- [CrossRef] [PubMed] [Google Scholar]
- SOX6 suppresses the development of lung adenocarcinoma by regulating expression of p53, p21(CIPI), cyclin D1 and b-catenin. FEBS Open Bio. 2020;10:135-46.
- [CrossRef] [PubMed] [Google Scholar]
- SOX13 exhibits a distinct spatial and temporal expression pattern during chondrogenesis, neurogenesis, and limb development. J Histochem Cytochem. 2006;54:1327-33.
- [CrossRef] [PubMed] [Google Scholar]
- SOX13 promotes colorectal cancer metastasis by transactivating SNAI2 and c-MET. Oncogene. 2020;39:3522-40.
- [CrossRef] [PubMed] [Google Scholar]
- Sex-determining Region Y-box transcription factor 13 promotes breast cancer cell proliferation and glycolysis by activating the tripartite motif containing 11-mediated Wnt/b-catenin signaling pathway. Bioengineered. 2022;13:13033-44.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations involving the SRY-related gene SOX8 are associated with a spectrum of human reproductive anomalies. Hum Mol Genet. 2018;27:1228-40.
- [CrossRef] [PubMed] [Google Scholar]
- High SOX8 expression promotes tumor growth and predicts poor prognosis through GOLPH3 signaling in tongue squamous cell carcinoma. Cancer Med. 2020;9:4274-89.
- [CrossRef] [PubMed] [Google Scholar]
- Over-expression of SOX8 predicts poor prognosis in colorectal cancer: A retrospective study. Medicine (Baltimore). 2019;98:e16237.
- [CrossRef] [PubMed] [Google Scholar]
- The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014;1:149-61.
- [CrossRef] [PubMed] [Google Scholar]
- SOX9 in organogenesis: Shared and unique transcriptional functions. Cell Mol Life Sci. 2022;79:522.
- [CrossRef] [PubMed] [Google Scholar]
- SOX9 is essential for triple-negative breast cancer cell survival and metastasis. Mol Cancer Res. 2020;18:1825-38.
- [CrossRef] [PubMed] [Google Scholar]
- SOX9 triggers different epithelial to mesenchymal transition states to promote pancreatic cancer progression. Cancers (Basel). 2022;14:916.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance of SOX10 expression in human pathology. Curr Issues Mol Biol. 2023;45:10131-58.
- [CrossRef] [PubMed] [Google Scholar]
- High expression of Sox10 correlates with tumor aggressiveness and poor prognosis in human nasopharyngeal carcinoma. Onco Targets Ther. 2016;9:1671-7.
- [CrossRef] [PubMed] [Google Scholar]
- SOX10 knockdown inhibits melanoma cell proliferation via notch signaling pathway. Cancer Manag Res. 2021;13:7225-34.
- [CrossRef] [PubMed] [Google Scholar]
- The transcription factor SOX7 modulates endocardiac cushion formation contributed to atrioventricular septal defect through Wnt4/Bmp2 signaling. Cell Death Dis. 2021;12:393.
- [CrossRef] [PubMed] [Google Scholar]
- SOX7 is down-regulated in lung cancer. J Exp Clin Cancer Res. 2013;32:17.
- [CrossRef] [PubMed] [Google Scholar]
- SOX7 inhibits tumor progression of glioblastoma and is regulated by miRNA-24. Open Med (Wars). 2016;11:133-7.
- [CrossRef] [PubMed] [Google Scholar]
- Role of SOX17 in hematopoietic development from human embryonic stem cells. Blood. 2013;121:447-58.
- [CrossRef] [PubMed] [Google Scholar]
- SOX17: A highly sensitive and specific immunomarker for ovarian and endometrial carcinomas. Mod Pathol. 2023;36:100001.
- [CrossRef] [PubMed] [Google Scholar]
- SOX17 inhibits tumor metastasis via wnt signaling in endometrial cancer. Onco Targets Ther. 2019;12:8275-86.
- [CrossRef] [PubMed] [Google Scholar]
- SOX18 affects cell viability, migration, invasiveness, and apoptosis in hepatocellular carcinoma (HCC) cells by participating in epithelial-to-mesenchymal transition (EMT) progression and adenosine monophosphate activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) Med Sci Monit. 2019;25:6244-54.
- [CrossRef] [PubMed] [Google Scholar]
- SOX18 promotes gastric cancer metastasis through transactivating MCAM and CCL7. Oncogene. 2020;39:5536-52. Erratum in: Oncogene 2021;40;4242-3
- [CrossRef] [PubMed] [Google Scholar]
- Effects of SOX15 on the colorectal cancer cells via downregulation of the Wnt/b-catenin signaling pathway. Future Oncol. 2018;14:1921-32.
- [CrossRef] [PubMed] [Google Scholar]
- Epigenetic inactivation of SOX30 is associated with male infertility and offers a therapy target for non-obstructive azoospermia. Mol Ther Nucleic Acids. 2020;19:72-83.
- [CrossRef] [PubMed] [Google Scholar]
- SOX30 is a prognostic biomarker and chemotherapeutic indicator for advanced-stage ovarian cancer. Endocr Relat Cancer. 2019;26:303-19.
- [CrossRef] [PubMed] [Google Scholar]
- SOX30 specially prevents Wnt-signaling to suppress metastasis and improve prognosis of lung adenocarcinoma patients. Respir Res. 2018;19:241.
- [CrossRef] [PubMed] [Google Scholar]
- The role of SRY-related HMG box transcription factor 4 (SOX4) in tumorigenesis and metastasis: Friend or foe? Oncogene. 2013;32:3397-409.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4: Epigenetic regulation and role in tumorigenesis. Semin Cancer Biol. 2020;67:91-104.
- [CrossRef] [PubMed] [Google Scholar]
- Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol. 2007;27:7802-15.
- [CrossRef] [PubMed] [Google Scholar]
- The role of SOX family members in solid tumours and metastasis. Semin Cancer Biol. 2020;67:122-53.
- [CrossRef] [PubMed] [Google Scholar]
- Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox-4. Nature. 1996;380:711-4.
- [CrossRef] [PubMed] [Google Scholar]
- Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sox4 links tumor suppression to accelerated aging in mice by modulating stem cell activation. Cell Rep. 2014;8:487-500.
- [CrossRef] [PubMed] [Google Scholar]
- TGF-β induces SOX2 expression in a time-dependent manner in human melanoma cells. Pigment Cell Melanoma Res. 2016;29:453-8.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 cooperates with Evi1 in AKXD-23 myeloid tumors via transactivation of proviral LTR. Blood. 2006;107:733-41.
- [CrossRef] [PubMed] [Google Scholar]
- Prolonged SOX4 expression in oligodendrocytes interferes with normal myelination in the central nervous system. Mol Cell Biol. 2007;27:5316-26.
- [CrossRef] [PubMed] [Google Scholar]
- The closely related transcription factors SOX4 and SOX11 function as survival factors during spinal cord development. J Neurochem. 2010;115:131-41.
- [CrossRef] [PubMed] [Google Scholar]
- Sequential requirement of Sox4 and Sox11 during development of the sympathetic nervous system. Development. 2010;137:775-84.
- [CrossRef] [PubMed] [Google Scholar]
- Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci U S A. 2004;101:9309-14.
- [CrossRef] [PubMed] [Google Scholar]
- Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res. 2006;66:4011-9.
- [CrossRef] [PubMed] [Google Scholar]
- Epigenetic repression of microRNA-129-2 leads to overexpression of SOX4 oncogene in endometrial cancer. Cancer Res. 2009;69:9038-46.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 induces epithelial-mesenchymal transition and contributes to breast cancer progression. Cancer Res. 2012;72:4597-608.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of Sox4 expression in human cutaneous melanoma and its role in cell migration and invasion. Am J Pathol. 2010;177:2741-52.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological significance of SOX4 expression in primary gallbladder carcinoma. Diagn Pathol. 2012;7:41.
- [CrossRef] [PubMed] [Google Scholar]
- Induction of SOX4 by DNA damage is critical for p53 stabilization and function. Proc Natl Acad Sci U S A. 2009;106:3788-93.
- [CrossRef] [PubMed] [Google Scholar]
- Knockdown of Sox4 expression by RNAi induces apoptosis in ACC3 cells. Oncogene. 2006;25:5626-39.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of SOX4 induces melanoma cell apoptosis via downregulation of NF-kB p65 signaling. Oncol Rep. 2018;40:369-76.
- [CrossRef] [Google Scholar]
- Down-regulated SOX4 expression suppresses cell proliferation, metastasis and induces apoptosis in Xuanwei female lung cancer patients. J Cell Biochem. 2015;116:1007-18.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 overexpression regulates the p53-mediated apoptosis in hepatocellular carcinoma: Clinical implication and functional analysis in vitro. Carcinogenesis. 2010;31:1298-307.
- [CrossRef] [PubMed] [Google Scholar]
- Overexpression of SOX4 promotes cell migration and invasion of renal cell carcinoma by inducing epithelial-mesenchymal transition. Int J Oncol. 2017;51:336-46.
- [CrossRef] [PubMed] [Google Scholar]
- Sox4 expression confers bladder cancer stem cell properties and predicts for poor patient outcome. Int J Biol Sci. 2015;11:1363-75.
- [CrossRef] [PubMed] [Google Scholar]
- Differential expression and prognostic significance of SOX genes in pediatric medulloblastoma and ependymoma identified by microarray analysis. Neuro Oncol. 2008;10:648-60.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 expression in bladder carcinoma: Clinical aspects and in vitro functional characterization. Cancer Res. 2006;66:3434-42.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 is associated with poor prognosis in prostate cancer and promotes epithelial-mesenchymal transition in vitro. Prostate Cancer Prostatic Dis. 2013;16:301-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and prognostic association of transcription factor SOX4 in gastric cancer. PLoS One. 2012;7:e52804.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and prognostic implications of transcription factor SOX4 in patients with colon cancer. PLoS One. 2013;8:e67128.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 promotes the growth and metastasis of breast cancer. Cancer Cell Int. 2020;20:468.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 contributes to the progression of cervical cancer and the resistance to the chemotherapeutic drug through ABCG2. Cell Death Dis. 2015;6:e1990.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 expression is associated with treatment failure and chemoradioresistance in oral squamous cell carcinoma. BMC Cancer. 2015;15:888.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 expression levels in urothelial bladder carcinoma. Pathol Res Pract. 2011;207:423-7.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of a progenitor gene program by SOX4 is essential for mammary tumor proliferation. Oncogene. 2021;40:6343-53.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 overexpression is a novel biomarker of malignant status and poor prognosis in breast cancer patients. Tumour Biol. 2015;36:4167-73.
- [CrossRef] [PubMed] [Google Scholar]
- Association of SOX4 regulated by tumor suppressor miR-30a with poor prognosis in low-grade chondrosarcoma. Tumour Biol. 2015;36:3843-52.
- [CrossRef] [PubMed] [Google Scholar]
- miR-129-2 suppresses proliferation and migration of esophageal carcinoma cells through downregulation of SOX4 expression. Int J Mol Med. 2013;32:51-8.
- [CrossRef] [PubMed] [Google Scholar]
- MiR-133a suppresses the migration and invasion of esophageal cancer cells by targeting the EMT regulator SOX4. Am J Transl Res. 2015;7:1390-403.
- [Google Scholar]
- SOX4 interacts with EZH2 and HDAC3 to suppress microRNA-31 in invasive esophageal cancer cells. Mol Cancer. 2015;14:24.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 is a potential prognostic factor in human cancers: A systematic review and meta-analysis. Clin Transl Oncol. 2016;18:65-72.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of SOX4 target genes using phylogenetic footprinting-based prediction from expression microarrays suggests that overexpression of SOX4 potentiates metastasis in hepatocellular carcinoma. Oncogene. 2008;27:5578-89.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 is a direct target gene of FRA-2 and induces expression of HDAC8 in adult T-cell leukemia/lymphoma. Blood. 2013;121:3640-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sox4 is a key oncogenic target in C/EBPa mutant acute myeloid leukemia [published correction appears in Cancer Cell 2014;25:257. Diruscio, Annalisa [corrected to Di Ruscio, Annalisa] Cancer Cell. 2013;24:575-88.
- [CrossRef] [PubMed] [Google Scholar]
- SOX4 enables oncogenic survival signals in acute lymphoblastic leukemia. Blood. 2013;121:148-55.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of SOX4 gene mutation in non-small cell lung cancer tissues. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2007;24:505-9.
- [Google Scholar]
- The SRY-HMG box gene, SOX4, is a target of gene amplification at chromosome 6p in lung cancer. Hum Mol Genet. 2009;18:1343-52.
- [CrossRef] [PubMed] [Google Scholar]
- miR-132 inhibits lung cancer cell migration and invasion by targeting SOX4. J Thorac Dis. 2015;7:1563-9.
- [Google Scholar]
- Increased expression of SOX4 is a biomarker for malignant status and poor prognosis in patients with non-small cell lung cancer. Mol Cell Biochem. 2015;402:75-82.
- [CrossRef] [PubMed] [Google Scholar]
- A crucial epithelial to mesenchymal transition regulator, Sox4/Ezh2 axis is closely related to the clinical outcome in pancreatic cancer patients. Int J Oncol. 2016;48:145-52.
- [CrossRef] [PubMed] [Google Scholar]
- Estrogen induces androgen-repressed SOX4 expression to promote progression of prostate cancer cells. Prostate. 2015;75:1363-75.
- [CrossRef] [PubMed] [Google Scholar]
- Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147-52.
- [CrossRef] [PubMed] [Google Scholar]
- hsa-miR-191 is a candidate oncogene target for hepatocellular carcinoma therapy. Cancer Res. 2010;70:8077-87.
- [CrossRef] [PubMed] [Google Scholar]
- MicroRNA-191 promotes pancreatic cancer progression by targeting USP10. Tumour Biol. 2014;35:12157-63.
- [CrossRef] [PubMed] [Google Scholar]
- miR-191 promotes tumorigenesis of human colorectal cancer through targeting C/EBPb. Oncotarget. 2015;6:4144-58.
- [CrossRef] [PubMed] [Google Scholar]
- MicroRNA-187, a downstream effector of TGFb pathway, suppresses Smad-mediated epithelial-mesenchymal transition in colorectal cancer. Cancer Lett. 2016;373:203-13.
- [CrossRef] [PubMed] [Google Scholar]
- miR-138 inhibits gastric cancer growth by suppressing SOX4. Oncol Rep. 2017;38:1295-302.
- [CrossRef] [PubMed] [Google Scholar]
- STAT3-mediated upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer metastasis by regulating SOX4. Mol Cancer. 2017;16:136.
- [CrossRef] [PubMed] [Google Scholar]
- Metformin inhibits epithelial-mesenchymal transition in prostate cancer cells: Involvement of the tumor suppressor miR30a and its target gene SOX4. Biochem Biophys Res Commun. 2014;452:746-52.
- [CrossRef] [PubMed] [Google Scholar]
- MicroRNA-25 suppresses proliferation, migration, and invasion of osteosarcoma by targeting SOX4. Tumour Biol. 2017;39:1010428317703841.
- [CrossRef] [PubMed] [Google Scholar]
- Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature. 2016;539:452-5.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating microRNAs in breast cancer: Novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017;8:e3045.
- [CrossRef] [PubMed] [Google Scholar]
- lncRNAUCA1 enhances cell proliferation through functioning as a ceRNA of Sox4 in esophageal cancer. Oncol Rep. 2016;36:2960-6.
- [CrossRef] [PubMed] [Google Scholar]
- An androgen receptor negatively induced long non-coding RNA ARNILA binding to miR-204 promotes the invasion and metastasis of triple-negative breast cancer. Cell Death Differ. 2018;25:2209-20.
- [CrossRef] [PubMed] [Google Scholar]
- Long non-coding RNA ABHD11-AS1 promotes colorectal cancer development through regulation of miR-133a/SOX4 axis. Biosci Rep. 2018;38:BSR20181386.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological significance of SOX4 and epithelialmesenchymal transition markers in patients with laryngeal squamous cell carcinoma. Medicine (Baltimore). 2021;100:e25028.
- [CrossRef] [PubMed] [Google Scholar]
- DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct Target Ther. 2021;6:254.
- [CrossRef] [PubMed] [Google Scholar]
- Epithelial to mesenchymal transition history: From embryonic development to cancers. Biomolecules. 2021;11:782.
- [CrossRef] [PubMed] [Google Scholar]
- The role of m6A RNA methylation in cancer. Biomed Pharmacother. 2019;112:108613.
- [CrossRef] [PubMed] [Google Scholar]
- Role of hypoxia in cancer therapy by regulating the tumor micro-environment. Mol Cancer. 2019;18:157.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of glycosphingolipids on cancer cell energy metabolism. Prog Lipid Res. 2020;79:101050.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol Ther. 2021;29:2185-208.
- [CrossRef] [PubMed] [Google Scholar]
- Pan-cancer analysis and experimental validation of SOX4 as a potential diagnosis, prognosis, and immunotherapy biomarker. Cancers (Basel). 2023;15:5235.
- [CrossRef] [PubMed] [Google Scholar]
- Multi-omics analysis of SOX4, SOX11, and SOX12 expression and the associated pathways in human cancers. J Pers Med. 2021;11:823.
- [CrossRef] [PubMed] [Google Scholar]






