Translate this page into:
Prostate cancer in India: Current perspectives and the way forward

*Corresponding author: Prashanth N. Suravajhala, Amrita School of Biotechnology, Amrita Vishwa Vidyapeetham, Kollam, Kerala, India. Bioclues.org, Hyderabad, India. prash@bioclues.org
-
Received: ,
Accepted: ,
How to cite this article: Bhargavi R, Khilwani B, Kour B, Shukla N, Aradhya R, Sharma D, et al. Prostate cancer in India: Current perspectives and the way forward. J Reprod Healthc Med 2023;4:8.
Abstract
Prostate cancer (PCa) is one of the burgeoning cancers worldwide. Of late, the number of cases in the Indian subcontinent has been doubling and the incidence as per the population-based cancer registries (PBCR) has increased at an alarming rate. In assessing the trends of PCa, there needs a statistical framework on incidence, comparing PBCRs vis-a-vis with hospital-based cancer registries not only to that of cohorts from India but also to that of West and other consortia. Our erstwhile pilot study on inferring whole-exome sequencing variants has yielded distinct polymorphisms in the Indian phenotype of PCa. There are impending challenges and gray areas that we discuss in this review in lieu of PCa pathogenesis and therapeutics.
Keywords
Prostate cancer
Challenges
Prostate-specific antigen
Therapeutics
Disparities
INTRODUCTION
Prostate cancer (PCa) is one of the burgeoning cancers worldwide presenting an upward trend in the global incidence rates [Figure 1a and b].[1] The population-based cancer registries (PBCRs) in India also depict an increasing trend in the age-adjusted incidence rates of PCa during the past decade [Figure 2a and b].[2,3] In assessing the trends of PCa, there needs a statistical framework on incidence, comparing PBCRs vis-a-vis with hospital-based cancer registries. From a total of 667,666 individuals, age-adjusted incidence rates were considered, and the prevalence of PCa and metastasis was tabulated to meet the sustainable development goals. However, the composite age and lifestyle in PCa-affected sub-populations are not largely studied or dealt with, and therefore, we provide a gist of statistics. Our previous collaborative works have yielded differentially expressed genes (DEGs) regulating the feed efficiency traits in eutherians.[4] What remained intriguing was that some of these eutherians turned out to be diabetic when overfed, which begged us to ask whether the DEGs are specific to reproductive tissues. Taken together, there could be a missing connection between a diet, largely vegetarian, and PCa. Our pilot study in the Indian cohort gave us very interesting results in the makeup of PCa, even as we underline the need to study whether or not vegetarians are protected against PCa.[5] This is also in agreement with Tantamango-Bartley et al. (2013) who had earlier shown that there is a low risk of PCa in vegan and strict vegetarian populations augmenting the fact that nutrition, diet, and, perhaps, epigenetics have a role to play in the pathogenesis of PCa.[6]
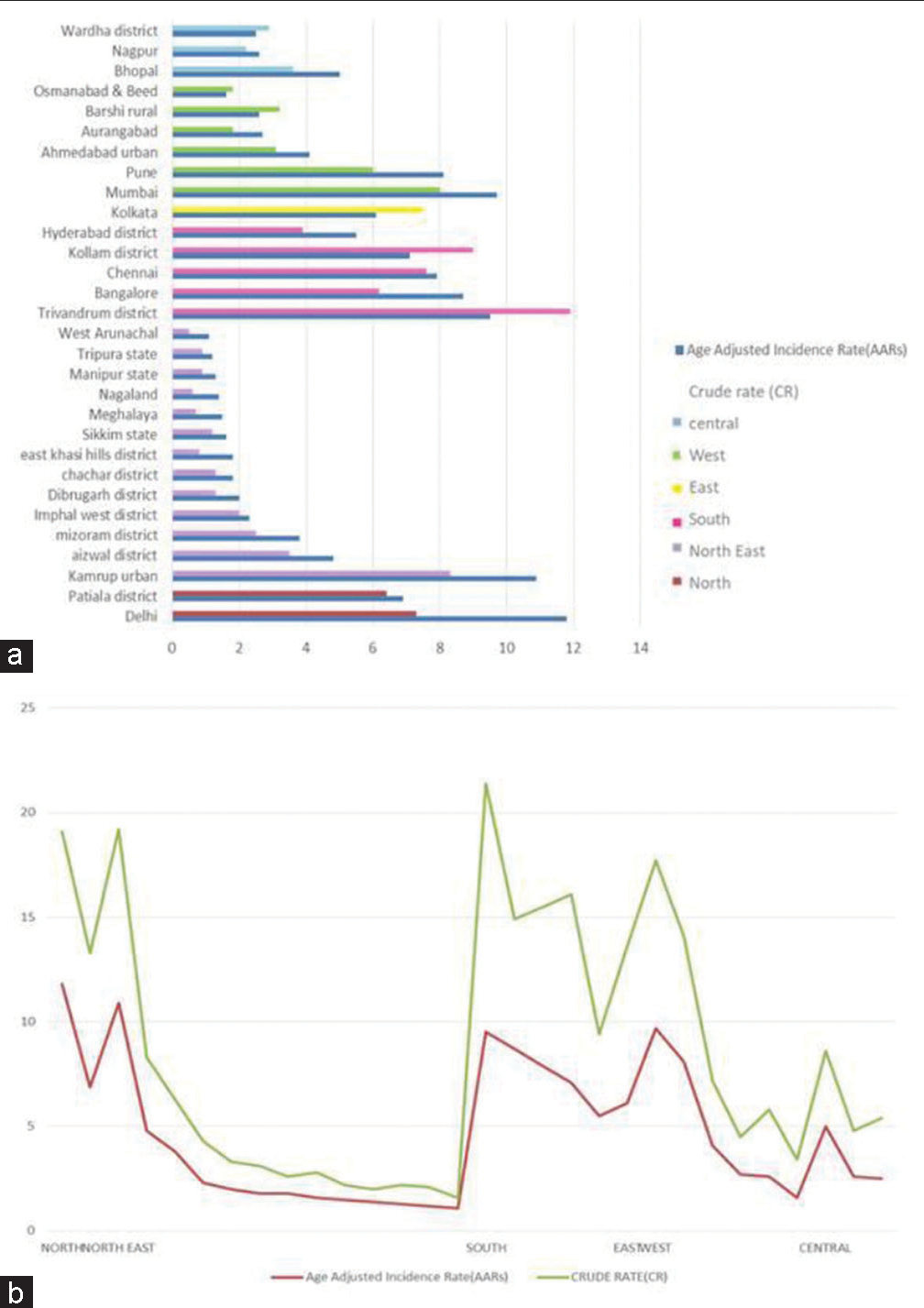
- (a) Comparison of age-adjusted incidence rates (AARs) and crude rates (CRs) of population-based cancer registries (PBCRs) for prostate cancer (PCa) till 2016-zone wise. (b) Comparison of AARs and CRs of PBCRs for PCa till 2016-district-wise.
The association of oxidative phosphorylation with diet needs to be explored particularly in a largely vegetarian population which is at a low risk of PCa and other cancers. Compared to a meat-based diet, vegetarian and fruit diets induce anti-oxidants in the body, which cleanse the intestine further enhancing cellular biogenesis. As PCa is non-cutaneous, this dietary pattern offers conclusively lower risk stratification not just in the Indian subpopulation but also across different races. For example, optic atrophy 1 (OPA1), a mitochondrial regulator, harbors distinct mutations and is known to be largely associated with cancer therapy and this very set of mutations was found in our pilot study.[5] We argue that OPA1 inhibition is essential for mitochondrial respiratory efficiency and is known to induce cell proliferation, thus serving as a hallmark for cancers.[7] PCa-related health disparities from an Indian perspective could largely be influenced by diet, lifestyle, environmental exposure, and climatic changes. Given the large influx of population from various states and immigrants into the Indian subcontinent, it is anticipated that genetic factors may influence the development of PCa where the androgen receptor (AR) signaling and hormonal pathways impede a chance for us to understand racial/ ethnic disparities as stated earlier. Nonetheless, India’s inter-racial subpopulation is influenced by PCa burden which could be due to the inherent stigma associated with these populations.
EPIDEMIOLOGY
Global scenario
PCa was reported to be the second most common cancer among men in 2020. According to the GLOBOCAN report, 2020,[8] and the number of new reported cases of PCa in 2020 was estimated at 1,414,259. The incidence rates worldwide are proportional to the human development index and gross domestic product, as it is believed that developed countries generally may have a higher incidence than developing nations. There is a three-fold difference in the incidence rates among countries, whereas mortality rates are less variable with incidence ranges between 6.3 and 83.4/100,000 men across regions [Figure 1]. The highest incidence rates were reported in Northern and Western Europe, the Caribbean, Australia/ New Zealand, Northern America, and Southern Africa, while the lowest rates were reported in Asia and Northern Africa. The mortality rates range from 3.1 to 27.9 with the Caribbean reporting the highest rate while south-central Asia reported the lowest. The variation in the global incidence rates can be attributed to the differences in awareness of PCa through access to diagnostic screening and screening frequency and differences in early diagnostic procedures adopted.[9] Higher incidence rates were reported in regions where preclinical detection of cancers was possible through prostate-specific antigen (PSA) testing.[10]
Indian scenario and disparities
Over the past decade, there has been a steady rise in PCa incidence in Asian countries especially in Singapore, China, Malaysia, and Japan possibly due to changes in diet and other lifestyle factors. The Indian scenario is still uncertain with respect to the true incidence of PCa due to the dearth of PBCRs and less than judicious reporting of cases, the inherent stigma attached to PCa, and a lack of community-based studies on PCa. The projected number of cases in India for 2020 was 51,979 as estimated by Jain et al.[2] According to the report by the National Cancer Registry Program-National Center for Disease Informatics and Research (NCDIR: https://ncdirindia.org/All_Reports/Report_2020/default.aspx last accessed on February 21, 2023) of Indian Council of Medical Research, Delhi-National Capital Region reported the highest age-adjusted incidence rate of PCa of over 11.8 cases/million adults registered followed by Kamrup region of Assam with ten cases/million adults and Thiruvananthapuram with nine cases/million adults between 2012 and 2016 [Figure 2].[2,3] The statewide disparities in the incidence rates of PCa in India can be attributed to lifestyle-related factors and the dearth of data collection and reporting of cases in rural areas.
PCa ETIOLOGY
PCa is one of the most common cancers worldwide; however, fairly little is known of its etiological factors. The major risk factors are advancing age, family cancer history, germline pathogenic variants, and single nucleotide variants associated with an increased PCa risk, ancestry, and mutations in certain genes (e.g., BRCA1 and BRCA2).[8] Age has a strong association with the risk of total PCa. The average age of PCa incidence in India is 65 years.[11] A growing body of evidence suggests that a family history of PCa increases its risk. Cases of positive family history where men with a father or brother with PCa have 2–3 times higher risk of being diagnosed with PCa.[12] Twin studies suggest familial aggregation of PCa through shared genetic factors with a high heritability estimate of 57%.[13,14] Genetic factors may be involved in early carcinogenesis as the germline risk loci studied are not strongly associated with lethal PCa.[15,16] An increased PCa mortality and incidence was reported in overweight and obese men with overweight men showing increased disease recurrence and worsened adverse effects to treatment. They also develop obesity-related comorbidities, which show earlier progression and development of metastatic disease and PCa-specific mortality. Despite the rise in cases, little is known of the underlying T mechanisms implicating obesity to PCa. A popular hypothesis suggests that metabolic abnormalities associated with adiposity create an inflammatory environment.[17] A recent theory postulates that PCa advances due to the state of lower cancer cell apoptosis, increased cancer cell growth, dysregulated angiogenesis, and finally metastasis and chemoresistance all of which can be triggered by the low-level chronic inflammation causing abnormal immune responses and metabolic aberrations.[18,19] Bacterial infection association with PCa risk and its subtle association with PSA biogenesis in augmenting cancer cannot be ruled out. For example, Roberts et al. have shown the link between tumor microenvironment (TME), inflammation, and (bacterial) prostatitis.[20] From a case–control study, they observed that on an average, acute prostatitis and its episodes are checked for early diagnosis of PCa risk which is ca. 12.2 years. However, chronic bacterial prostatitis is weakly correlated with PCa risk, thereby suggesting that the infection in the form of acute or chronic prostatitis may be associated with PCa. However, PCa pathogenesis is also attributed to the pleiotropic nature of genes and their expression, the development of organoids, and the risk of bacterial infection and its effect on PCa.
COMPARISON OF PCa RISK SINGLE-NUCLEOTIDE POLYMORPHISMS (SNPS) BETWEEN CANCER PROSTATE CONSORTIUM OF INDIA (CAPCI) AND PRACTICAL CONSORTIA
PCa is a life-threatening polygenic disease, associated with approximately 60% of heritability and more than 100 SNPs pose a major risk in the development of PCa. Over the years, studies have identified several SNPs that are associated with a higher risk of developing PCa. These SNPs are located in genes involved in regulating cell growth and division, DNA repair, and inflammation. Some of the most well-known risk SNPs for PCa in the Asian Indian cohort from genome-wide association studies (GWAS) include rs16901979 and rs1447295 in the 8q24 region, rs10896449 in the 17q12 region, and rs6983267 in the 10q11 region.[21,22] However, it is important to note that having a risk SNP does not guarantee that an individual will develop PCa. Other factors, such as age, family history, and lifestyle, also play a role in determining a person’s overall risk of developing the disease. Nonetheless, not all SNPs associated with PCa have the same level of risk, for example, some SNPs have a relatively small effect on risk, while others have a more substantial impact. It is also important to consider that different populations may have different frequencies of these SNPs, which can affect their overall impact on disease risk. Taken together, understanding the role of risk SNPs in PCa can help identify individuals who may be at an increased risk of developing the disease. However, more research is needed to fully understand the complex interplay between genetic and environmental factors in the development of PCa. Our erstwhile pilot study yielded a good representation of PCa risk SNPs and we sought to ask whether or not any SNPs are common between CAPCI and PCa Association Group to Investigate Cancer-Associated Alterations in the Genome (PRACTICAL) Consortia (bioclues.org/capci and http://practical.icr.ac.uk/ last accessed on February 22, 2023). We found four common SNPs between CAPCI and PRACTICAL Consortium’s Oncoarray data and 12 common SNPs between PRACTICAL Oncoarray and GWAS study.[5] The SNPs uncovered were run through the SNP databases in NCBI and minor allele frequencies (MAFs) of the SNPs in various subpopulations were noted as represented with mean MAF cutoff <0.05 for Oncoarray and <0.1 for GWAS used for screening the candidates [Figure 3]. The variant allele frequency, however, could be probed to check the heterogeneity and individual tumor burden associated with the pathogenicity.
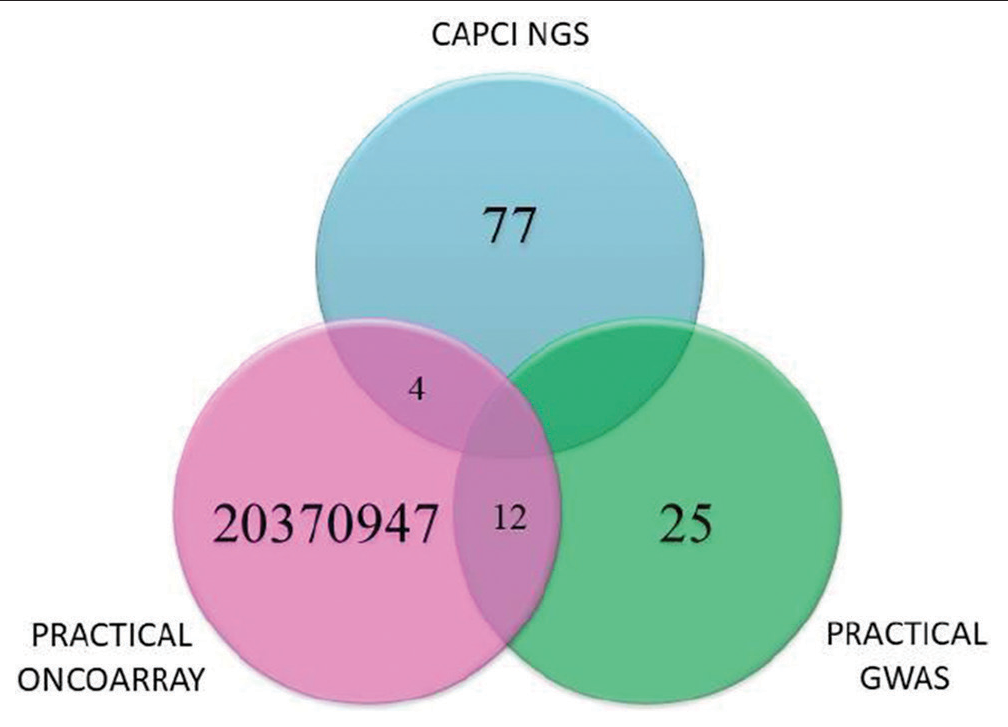
- Comparison of risk single-nucleotide polymorphisms between PRACTICAL Consortium and Cancer prostate consortium of India.
ARs and their associations in PCa
Medications that can selectively target tumors while avoiding non-target areas employ homing devices termed ligands. These include any molecule that recognizes and binds to target antigen or receptors overexpressed or selectively expressed by particular cells or tissue components. An ideal ligand for targeted delivery would be one with high avidity; specificity of binding to the cell surface receptors; should stimulate the internalization of polymeric particles that need internalization for its intracellular action; should be compatible with chemical modification for conjugation; and can be produced in sufficient quantity. AR is a member of the superfamily of ligand-dependent nuclear transcription factors. AR regulates multiple cellular events such as proliferation, apoptosis, migration, invasion, and differentiation. It is expressed in primary PCa cells as well as in metastases cases. Two main ligands that bind and activate the AR are testosterone and its metabolite dihydrotestosterone.
Testosterone plays a significant role in normal growth and function of the prostate gland. PCa is considered an androgen-dependent cancer as the levels of androgens regulate the growth of both normal and cancer prostate cells by binding and activating the AR.[23] Before any treatment, at diagnosis, serum testosterone value is a predictor of disease aggressiveness – lower testosterone values are related to less differentiated cancer and worse prognosis. Serum testosterone levels decline in all men with age and this androgen deficiency known as hypogonadism leads to symptoms such as decreased muscle mass, decreased energy levels, and erectile dysfunction. Testosterone replacement therapy (TRT) is utilized in hypogonadal cases where exogenous testosterone and other agents are administered. Although the popularity of TRT has substantially increased in recent times among older males, there is a potential risk of an increase in PCa incidence. Progression of PCa is hindered by androgen depletion and exogenous testosterone administration might stimulate metastatic PCa growth. TRT increases serum-free testosterone levels; thus, the development of clinically significant PCa needs to be monitored carefully in older men receiving TRT. One observation says that PCa is exquisitely sensitive to variations in androgens at low concentrations and others suggest that PCa is indifferent to variations at normal and high concentrations of androgens. These observations have replaced the commonly known “androgen hypothesis” with a saturation model implicating that PCa is sensitive to changes in androgen at lower concentrations (i.e., androgen-dependent) but does not respond to changes in androgen concentrations above the saturation point (androgen-independent). Current evidence suggests that the saturation point for human prostate tissue is approximately 250 ng/dL (approximately 8 nmol/L).[24] The saturation point may differ between individuals and vary considerably between tissue types.[25]
PCa THERAPEUTICS
New treatments are required for PCa because, despite decades of advances, PCa remains the second most common cause of cancer-related death in men. The current treatment options for PCa, including surgery, radiation therapy, chemotherapy, and hormonal therapy, have limitations and may not always be effective, especially in advanced stages of the disease. Furthermore, some treatments may cause significant side effects that impact the quality of life of patients. Therefore, the development of new and more effective treatments is necessary to improve patient outcomes and reduce the morbidity and mortality associated with PCa.
Prostate-specific membrane antigen (PSMA)-targeted therapies
PSMA is a protein that is overexpressed in PCa cells. PSMA is a type II integral membrane glycoprotein that was first detected in the human prostatic carcinoma cell line LNCaP.[26] PSMA is upregulated in the majority of PCa, and thus has been explored as a promising new target for specific imaging of PCa. There are now several PSMA-targeted therapies in development, including radioligand therapies and antibody-drug conjugates. At present, several small-molecule PSMA ligands with excellent in vivo tumor targeting characteristics are being investigated for their potential androgen theranostic applications in PCa. Signaling of PSMA has been linked with AR signaling, PI3K/Akt, and DDR signaling. The chemotherapeutic application of 177Lu-PSMA has recently been identified as successful in causing tumor shrinkage and delay in disease progression, specifically in metastatic castration-resistant PCa (mCRPC).[27] Immunotherapies targeting PSMA, small molecule ligands for PSMA, and some antibody therapies are under different stages of clinical trials implying targeted tumor reduction in early PCa cases. PSMA-targeted therapies have exhibited encouraging antitumor activities in PCa.[28] The most used treatment among different types of adoptive cellular therapy is chimeric antigen receptor (CAR)-T-cell therapy which presently is in clinical trials. PSMA is the most reliable target for CAR-T-cell therapy.[29] PSMA-directed radioligand therapy prolongs overall survival in men with mCRPC. Recent advances imply that PSMA-based therapies will become a standard of care in the management of patients with PCa. Over the years, various nanoparticle systems have been employed for theranostics. For example, lutetium-177-PSMA-617 and antibody-drug conjugates like BAY 2731954. These therapies have shown promising results in early clinical trials.[28,30] On the other hand, the use of AR pathway inhibitors, such as enzalutamide and abiraterone, has improved overall survival in patients with mCRPC.[31] Similarly, the use of PARP inhibitors, such as olaparib and rucaparib, has shown promise in patients with metastatic castration-sensitive PCa with DNA repair gene mutations.[32] Precision medicine approaches can also be used to develop more effective screening and prevention strategies for PCa. For example, genetic testing can identify men at increased risk of PCa due to inherited genetic mutations, such as mutations in the BRCA1 or BRCA2 genes.[33] These men can then be offered more intensive screening or prophylactic surgery to reduce their risk of developing PCa.
Immunotherapy combinations
In PCa, immunotherapy has shown promise as a potential treatment option, particularly in patients with advanced disease. One approach to immunotherapy in PCa is the use of checkpoint inhibitors, which target proteins that normally regulate immune system activity. The Programmed Cell Death-1/Programmed Cell Death Ligand-1 (PD-1/PD-L1) pathway is one such checkpoint that is often upregulated in cancer cells, leading to the suppression of immune responses. Antibodies that block either PD-1 or PD-L1 have been shown to enhance T-cell activity and improve outcomes in several types of cancer, including melanoma, lung cancer, and bladder cancer. Several clinical trials have investigated the use of checkpoint inhibitors in PCa, with mixed results. In a phase 3 trial (KEYNOTE-199), the PD-1 inhibitor pembrolizumab was combined with chemotherapy in patients with mCRPC. While the combination did not significantly improve overall survival compared to chemotherapy alone, there was a trend toward improved survival in patients with high PD-L1 expression on tumor cells.[34] Another phase 3 trial (IMbassador250) evaluated the PD-L1 inhibitor atezolizumab in combination with enzalutamide (an AR inhibitor) in patients with mCRPC. The combination did not significantly improve overall survival compared to enzalutamide alone but did improve radiographic progression-free survival.[35] Besides this, several trials are investigating other immunotherapy approaches in PCa in lieu of vaccines and adoptive T-cell therapy. It is important to note that immunotherapy is not yet a standard treatment option for PCa, and further, “research is needed to formulate an approach with optimum benefits to the patients.
Genomic profiling
In PCa, gene profiling can be used to identify the specific molecular pathways that are involved in the development and progression of the disease. This information can be used to develop personalized treatment plans that target the specific genetic mutations that are driving the cancer. There are several different gene profiling techniques that can be used in PCa, including microarray analysis and RNA sequencing. Microarray analysis involves measuring the expression levels of thousands of genes simultaneously, while RNA sequencing allows for a more detailed analysis of the transcriptome. Several studies have explored the use of gene profiling in PCa, with promising results. One example of gene profiling in PCa is the use of the Prolaris test, which analyzes the expression levels of genes associated with cell proliferation. This test has been shown to be a valuable tool for predicting the risk of PCa recurrence and guiding treatment decisions for patients with localized PCa.[36] Another example of gene profiling in PCa is the use of the Oncotype DX test, which analyzes the expression levels of genes associated with tumor aggressiveness. This test has been shown to be a valuable tool for predicting the risk of PCa recurrence and guiding treatment decisions for patients with intermediate-risk PCa.[37] In addition to guiding treatment decisions, gene profiling can also be used to identify potential therapeutic targets for PCa. For example, a study published in cancer research identified a gene signature associated with PCa bone metastasis, which may lead to the development of new treatments targeting these specific genes.[38] Overall, gene profiling holds great promise as a therapeutic option in PCa, as it can provide valuable information for developing personalized treatment plans and monitoring treatment response. However, further research is needed to fully explore the potential of this technique in the management of PCa.
Liquid biopsies
In PCa, liquid biopsies have become increasingly important as a tool for disease diagnosis, monitoring, and prognosis. Liquid biopsy-based tests can provide clinicians with real-time information on cancer progression and treatment response, which can inform clinical decision-making. There are several types of liquid biopsy tests for PCa, including circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), and exosomes. The CTCs are tumor cells that have broken away from the primary tumor and entered the bloodstream. ctDNA is released into the bloodstream when tumor cells die and break apart, and exosomes are small membrane-bound vesicles that are released by tumor cells and carry tumor-specific molecules.[39] In PCa, liquid biopsies have been shown to have several clinical applications, including early detection. Studies have shown that liquid biopsies can detect PCa with a sensitivity and specificity of over 90%. It can be used to monitor treatment response and detect treatment resistance. For example, studies have shown that ctDNA levels can predict treatment response to chemotherapy and androgen deprivation therapy.[40] It can also provide information on tumor aggressiveness and predict disease progression. For example, studies have shown that the presence of CTCs and high levels of ctDNA are associated with poor prognosis.[41] There are several liquid biopsy-based tests currently available for PCa, including the Guardant360 test, the Oncotype DX PCa test, and the ExoDx Prostate IntelliScore test. These tests have been shown to have high accuracy in detecting PCa and predicting treatment response.
Taken together, these developments represent promising avenues for the development of new and more effective treatments for PCa. Advances in genomic profiling and targeted therapies have shown promise in improving treatment outcomes and reducing side effects. Further, research is needed to validate the clinical utility of precision medicine approaches and to identify additional molecular targets for therapy.
CHALLENGES IN PCa GENOMICS
Tumor heterogeneity
PCa is a heterogeneous disease, and tumors can differ significantly in terms of genetic alterations and gene expression profiles. This heterogeneity can make it challenging to identify genetic drivers of PCa and to develop targeted therapies.
Lack of comprehensive genomic data
Despite significant advances in PCa genomics, there is still a lack of comprehensive genomic data on this disease. This is partly due to the difficulty in obtaining high-quality prostate tumor samples, which can be contaminated with normal tissue.
Lack of representative models
The lack of representative in vitro and in vivo models that accurately reflect the complexity and heterogeneity of PCa also poses a challenge. This hampers the ability to test the efficacy and safety of potential therapies in preclinical models.
Limited understanding of the functional significance of genomic alterations
Although many genetic alterations have been identified in PCa, their functional significance is often unclear. This can make it difficult to develop targeted therapies based on these alterations.
Inter-patient variability
PCa can vary significantly from patient to patient, making it difficult to develop personalized treatment strategies based on genomic data.
Evolution of the disease
PCa is a disease that evolves over time, with tumors accumulating new mutations and genetic alterations as they progress. This can make it challenging to identify the most relevant genetic alterations for targeted therapies.
Lack of diversity in genomic studies
Many genomic studies of PCa have focused on patients of European ancestry, which can limit the generalizability of findings to other populations. There is a need for more diverse genomic studies of PCa.
CONCLUSION
Over the years, advancements in medical diagnostic and therapeutic techniques have allowed for translational practices in the emerging areas of sexual and reproductive health. Many professional societies have been instrumental in developing a framework to disseminate findings in the areas, viz. (a) Societal awareness for PCa genetic testing which would bridge the gap between society and geneticists, (b) cataloging candidate mutations to develop a multigene panel for testing PCa, (c) therapeutic targets for facilitating precision medicine, (d) one health regime, and (e) implementation of high throughput technologies. Genetic screening is used to identify individuals who have genetic variations. Awareness of PCa risk is a deciding factor for genetic screening and testing which may be useful to plan for periodic evaluation, implementation of preventive strategies, or initiation of therapeutic interventions. Hereditary PCa genetic testing criteria are based on an individual’s family history, personal/disease characteristics, and tumor sequencing results. We should forge ahead in bringing advancements in genetic testing for cancers including PCa that will have far greater benefits, and less harm when done in conjunction with well-designed health education and enliven the experiences. Long hail healthy genomes!
Authors’ contributions
BR and BK wrote the first draft. PNS and NKL conceptualized the perspective. Other authors chipped in with lateral sections. PNS proofread the manuscript before all authors agreed to the final version.
Data availability
Not Applicable.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
Nirmal Kumar Lohiya is the member of the Editorial Board.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Prostate cancer incidence and mortality: Global status and temporal trends in 89 countries from 2000 to 2019. Front Public Health. 2022;10:811044.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of prostate cancer in India. Meta Gene. 2014;2:596-605.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer statistics, 2020: Report from national cancer registry program, India. JCO Glob Oncol. 2020;6:1063-75.
- [CrossRef] [PubMed] [Google Scholar]
- From genetical genomics to systems genetics: Potential applications in quantitative genomics and animal breeding. Mamm Genome. 2006;17:548-64.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot study on the whole exome sequencing of prostate cancer in the Indian phenotype reveals distinct polymorphisms. Front Genet. 2020;11:874.
- [CrossRef] [PubMed] [Google Scholar]
- Vegetarian diets and the incidence of cancer in a low-risk population cancer and vegetarian diets. Cancer Epidemiol Biomarkers Prev. 2013;22:286-94.
- [CrossRef] [PubMed] [Google Scholar]
- OPA1, a new mitochondrial target in cancer therapy. Aging (Albany NY). 2020;12:20931-3.
- [CrossRef] [PubMed] [Google Scholar]
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- Prostate cancer health disparities: An immuno-biological perspective. Cancer Lett. 2018;414:153-65.
- [CrossRef] [PubMed] [Google Scholar]
- International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079-92.
- [CrossRef] [PubMed] [Google Scholar]
- Estimation of time trends of incidence of prostate cancer-an Indian scenario. Asian Pac J Cancer Prev. 2012;13:6245-50.
- [CrossRef] [PubMed] [Google Scholar]
- Attributable risks of familial cancer from the Family-Cancer Database. Cancer Epidemiol Biomarkers Prev. 2002;11:1638-44.
- [Google Scholar]
- Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78-85.
- [CrossRef] [PubMed] [Google Scholar]
- Familial risk and heritability of cancer among twins in Nordic countries. JAMA. 2016;315:68-76.
- [CrossRef] [PubMed] [Google Scholar]
- Genetics of prostate cancer risk. Mt Sinai J Med. 2010;77:643-54.
- [CrossRef] [PubMed] [Google Scholar]
- Prostate cancer (PCa) risk variants and risk of fatal PCa in the national cancer institute breast and prostate cancer cohort consortium. Eur Urol. 2014;65:1069-75.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity and prostate cancer: A narrative review. Crit Rev Oncol Hematol. 2022;169:103543.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421-49.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanistic insights into the link between obesity and prostate cancer. Int J Mol Sci. 2021;22:3935.
- [CrossRef] [PubMed] [Google Scholar]
- Prostatitis as a risk factor for prostate cancer. Epidemiol. 2004;15:93-9.
- [CrossRef] [PubMed] [Google Scholar]
- Androgen receptor CAG repeat polymorphism and risk of TMPRSS2: ERG-positive prostate cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:2027-31.
- [CrossRef] [PubMed] [Google Scholar]
- Two independent prostate cancer risk-associated loci at 11q13. Cancer Epidemiol Biomarkers Prev. 2009;18:1815-20.
- [CrossRef] [PubMed] [Google Scholar]
- The role of the androgen receptor in prostate development and benign prostatic hyperplasia. Asian J Urol. 2020;7:191-202.
- [CrossRef] [PubMed] [Google Scholar]
- Serum PSA as a predictor of testosterone deficiency. J Sex Med. 2013;10:2518-28.
- [CrossRef] [PubMed] [Google Scholar]
- Shifting the paradigm of testosterone and prostate cancer: The saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55:310-20.
- [CrossRef] [PubMed] [Google Scholar]
- Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81-5.
- [Google Scholar]
- [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825-33.
- [CrossRef] [Google Scholar]
- EANM procedure guidelines for radionuclide therapy with 177 Lu-labelled PSMA-ligands (177 Lu-PSMARLT) Eur J Nucl Med Mol Imaging. 2019;46:2536-44.
- [CrossRef] [PubMed] [Google Scholar]
- Chimeric antigen receptor T-cell therapy in metastatic castrate-resistant prostate cancer. Cancers (Basel). 2022;14:503.
- [CrossRef] [PubMed] [Google Scholar]
- TROPiCS-04: Study of sacituzumab govitecan in metastatic or locally advanced unresectable urothelial cancer that has progressed after platinum and checkpoint inhibitor therapy. J Clin Oncol. 2021;39:TPS498.
- [CrossRef] [Google Scholar]
- Abiraterone and other novel androgen-directed strategies for the treatment of prostate cancer: A new era of hormonal therapies is born. Ther Adv Urol. 2012;4:167-78.
- [CrossRef] [PubMed] [Google Scholar]
- DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-708.
- [CrossRef] [PubMed] [Google Scholar]
- Re: PROREPAIR-B: A prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37:490-503.
- [CrossRef] [PubMed] [Google Scholar]
- KEYNOTE-641: A phase III study of pembrolizumab plus enzalutamide for metastatic castration-resistant prostate cancer. Future Oncol. 2021;17:3017-26.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): Results from a randomised, phase 3 trial. Lancet Oncol. 2015;16:509-21.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol. 2013;31:1428-34.
- [CrossRef] [PubMed] [Google Scholar]
- A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low-and intermediate-risk prostate cancer. Eur Urol. 2015;68:123-31.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of key gene signatures associated with bone metastasis in castration-resistant prostate cancer using Co-expression analysis. Front Oncol. 2021;10:571524.
- [CrossRef] [PubMed] [Google Scholar]
- Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J Natl Cancer Inst. 2017;110:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7:312re10.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating tumor cells in peripheral blood samples from patients with increased serum prostate specific antigen: Initial results in early prostate cancer. J Urol. 2008;179:2187-91.
- [CrossRef] [PubMed] [Google Scholar]



![(a) Age-standardized (world) incidence and mortality rates for prostate cancer (PCa) as per GLOBOCAN, 2020.[8] (b) Crude rate and mortality risk of PCa worldwide as per GLOBOCAN, 2020.[8]](/content/117/2023/4/1/img/JRHM-4-8-g001.png)




