Translate this page into:
Role of genetic, environmental, and hormonal factors in the progression of PCOS: A review

*Corresponding author: Rakesh Kumar, School of Biotechnology, Shri Mata Vaishno Devi University, Katra, Jammu and Kashmir, India. kumar.rakesh@smvdu.ac.in
-
Received: ,
Accepted: ,
How to cite this article: Kumar R, Minerva S, Shah R, Bhat A, Verma S, Chander G, et al. Role of genetic, environmental, and hormonal factors in the progression of PCOS: A review. J Reprod Healthc Med 2022;3:3.
Abstract
Polycystic ovary syndrome (PCOS) can be mainly defined as a gynecological problem accompanied by an endocrine disturbance in females and can be seen mainly during their menstruation age. Worldwide PCOS prevalence ranges between 6% and 10%. Many of the risk factors are responsible for the pathogenesis of PCOS. Environmental factors such as environmental toxins and obesity play a major role in the occurrence of PCOS, followed by the hormonal disturbance in androgen levels, that is, hyperandrogenism, and insulin levels, that is, hyperinsulinemia. The previous studies have suggested that there is a major contribution of genetics in the etiology of PCOS. However, there is no strong evidence about the mode of inheritance of PCOS. It has been seen that there is a strong correlation between environmental, hormonal, and genetic factors which follow a vicious cycle in the development of PCOS that leads to ovarian dysfunction, metabolic syndrome, that is, metabolic abnormalities include insulin resistance, obesity, hypertension, dyslipidemia, and abnormal cholesterol level. The detailed study of PCOS is one of the most central topics in female reproductive endocrinology. On the other hand, the syndrome has been extensively investigated; however, its definition and pathophysiological aspects are still not very clear. The idea behind the current review was to make a non-systematic review of already published literature through PubMed and Google Scholar search. The keywords searched and publications were related to polycystic ovaries, including the incidence, environmental factors, genetics, hormones, as well as their association. The aim of the current review is to update the evidence regarding the pathogenesis of PCOS and emphasizes how genetic, non-genetic, and hormonal factors lead to the progression of PCOS.
Keywords
PCOS
Incidence
Environmental Factors
Genetic Factors
Hormonal Factors
INTRODUCTION
Polycystic ovary syndrome (PCOS) can be defined as a complex combination of chronic anovulation (menstrual dysfunction), hyperandrogenism, and gonadotropin abnormalities with the presence of polycyst in the ovaries.[1] Anovulation is basically a menstrual dysfunction followed by amenorrhea, oligomenorrhea, and dysfunctional uterine bleeding, which is the most common cause of infertility. Most women suffering from PCOS are only diagnosed when seeking infertility treatment. Women with PCOS face difficulties in conceiving, resulting in primary or secondary infertility. Hyperandrogenism is mainly related to the overproduction of androgen hormone which can be identified by hirsutism, acne, and obesity.[2] Gonadotropin abnormalities mainly arise due to defects in the gonadotropin secretions which cause an imbalance in the luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels, leading to a rise in the LH and FSH ratio.[3] PCOS is a multigenic disease involving discrete genes, and interaction between genes, the interconnection between genes and surroundings has been seen, which influences the development of PCOS. Although the definite reason for PCOS is not very clear; according to studies, the association of insulin resistance, obesity, diabetes mellitus type 2, dyslipidemia, metabolic syndrome, hypertension, cardiovascular disease, hyperplasia, and endometrial carcinoma has seen with the syndrome.[4] A detailed study of PCOS is one of the most central topics in female reproductive endocrinology. Therefore, the current review aims to update the evidence regarding the pathogenesis of PCOS and emphasizes how genetic, non-genetic, and hormonal factors lead to the progression of PCOS.
INCIDENCE
The frequency of PCOS has increased over the past years and in 2020, it is estimated that the prevalence of PCOS worldwide ranges between 6% and 10%.[5] Among the Asian population, the prevalence of PCOS is widely varying. It is estimated that the prevalence in China is 10.1%.[6] The prevalence in Thailand is 1.81%.[7] Iran is about 3%.[8] Pakistan is about 20%.[9] As compared to western women, Indian women have a higher prevalence of PCOS with a range of 3.7–22.5%.[10]
RISK FACTORS ASSOCIATED WITH PCOS
Various risk factors are responsible for the development of PCOS including genetic factors, non-genetic factors, and hormonal factors [Figure 1].
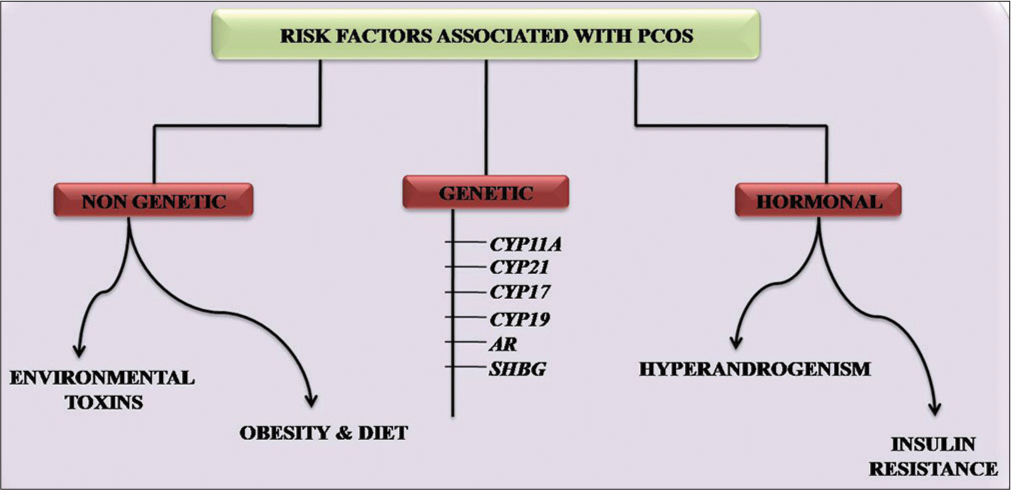
- Risk factors associated with PCOS. PCOS: Polycystic ovary syndrome.
Non-genetic factors
Several lifestyle factors and environmental toxins play a key role in the pathophysiology of PCOS.
Environmental Toxins: Environmental toxins refer to the chemical pollutants present in the environment. These chemical pollutants can enter the human body by inhalation or absorption by the skin and mucous membranes.[11] One of the common toxins is endocrine-disrupting chemicals such as tobacco, lead, mercury, pesticides, and industrial pollutants which have the potential to interfere with the hormone-sensitivity system.[11] There are shreds of evidence that show significant and longer effects of these toxins on reproductive health.[12]
Obesity and Diet: Obesity has been considered a risk factor contributing to PCOS. It is considered the major threat around the world due to the increased prevalence among women. It is the result of diet, stress, and sedentary life which later affects the reproductive process.[13] Obesity in PCOS women increases the risk of hyperandrogenism, hirsutism, insulin resistance, and infertility.[14,15]
Genetic factors
Several studies have suggested a major contribution of genetic factors in the etiology of PCOS. However, no strong evidence regarding the mode of inheritance of PCOS has been reported yet. A study by Kahsar-Miller et al. suggested that first-degree relatives of PCOS patients are at higher risk of developing PCOS.[16] Many candidate genes are involved in the biosynthesis and metabolism of ovarian steroidogenesis. Disturbance in the transcription of these genes leads to the up-regulation of androgen levels. Genes are mainly involved in the ovarian steroidogenesis of PCOS which includes CYP11A, CYP21, CYP17, CYP19, AR, and sex hormone-binding globulin (SHBG).[17]
CYP11A: CYP11A encodes the enzyme P450 which is key for the synthesis of cholesterol, steroids, lipids, and drug metabolism. The enzyme catalyzes the reaction by converting cholesterol to progesterone. This step is the main rate-restricting step involved in androgen hormone synthesis. Gharani et al. have reported an association of CYP11A gene with hyperandrogenism and PCOS.[18] Several studies were conducted in several areas of the world which concluded the association of PCOS progression with CYP11A locus having repetitive sequence TTTTA at the 5’UTR region.[19] Furthermore, Zhang et al. studied the significant association of SNP rs4077582 in CYP11A with PCOS development along with the altered level of androgen.[20]
CYP21: CYP21 encodes the enzyme P45021-hydroxylase that converts 17-hydroxyprogesterone into 11-deoxycortisol. The inactivation of P45021-hydroxylase leads to the futile anabolism of steroidogenesis.[21] According to a recent study, the association of the CYP21 with excessive androgen levels has been reported.[22]
CYP17: CYP17 encodes the enzyme P450 17-hydroxylase/17, 20-desmolase required for the 17-hydroxylase activity. It converts pregnenolone into 17-hydroxypregnenolone and progesterone into 17-hydroxyprogesterone. According to a study, CYP17 contributes to the abnormal androgen level and development of PCOS.[23] Further, a study revealed the association of an unusual T/C single nucleotide polymorphism at the promoter region of CYP17 contributes to the abnormal androgen level and development of PCOS.[23] Later, the study by Pusalkar et al. concluded that Indian women have a high frequency of allele C with PCOS and are prone to develop hyperandrogenic features among them.[24]
CYP19: CYP19 is responsible for the aromatase, essential for the formation of estrogens.[25] According to the study, a decrease in aromatase activity has been seen in obese and lean women having PCOS, accompanied by a decreased androgen level.[26] A study was conducted to check the polymorphism of CYP19 and SNP rs2470152 and concluded the association of heterozygous TC with an elevated level of testosterone which is involved in the aromatase activity.[20] Furthermore, the study was conducted to check the association of different SNPs rs700519, rs60271534, and rs2414096, which concluded the significant association with PCOS development in the Indian population.[27]
AR: AR is also an Androgen receptor gene. AR is present on the X chromosome. It consists of a weakly associated N terminal domain having extreme CAG repeats.[22] Several studies have shown the role of CAG repeats and AR which concludes the elevated frequencies of AR and CAG repeats among PCOS women.[28,29] Furthermore, according to the study, inactivation in the X chromosome causes disturbance in the normal cellular mechanisms that lead to an increased level of androgen which leads to PCOS.[25]
SHBG: SHBG encoded protein products that maintain the sex hormones levels, and binds with androgen, estrogens, and testosterone with high affinity.[30] The synthesis of sex hormones is managed by several metabolic factors, that is, insulin and androgen.[31,32] According to a recent study, there is a significant association between single nucleotide polymorphism and the SHBG.[33,34] Studies suggest that there are shreds of evidence of SHBG polymorphism which shows the association of long TAAAA repeats with prolonged menarche.[26]
Besides that, PCOS can be considered a kind of ovary functional hyperandrogenism. As the above-mentioned, abbreviated genes contribute toward not only ovarian steroidogenesis but also cause reproductive dysfunctions along with metabolic abnormalities seen in women with PCOS [Figure 2].[26]

- The outline of pathophysiology of PCOS demonstrates the biosynthesis of androgen. PCOS: Polycystic ovary syndrome.
Hormonal factors
Hyperandrogenism: Hyperandrogenism can be defined as the classic alteration in the normal ovarian function which results in the overproduction of androgen.[35] Hyperandrogenism can be confirmed by calculating the levels of total serum testosterone, sex hormone binding globulin SHBG, 17-hydroxy progesterone, and free androgen index.[36] Although multiple factors are involved in the origin of hyperandrogenism which is mainly attributed to the ovary along with adrenal dysfunction and lesser influence from fatty tissues. Most women with PCOS have high levels of LH and low FSH during their menstrual phase. The disturbance in the normal phenomenon leads to the elevation in the level of LH that promotes the production of androgen which detains the follicular development and the drop in the FSH promotes the growth of small follicles.[37,38]
Insulin Resistance: Insulin resistance can be defined as the inability of a cell to respond well to insulin either to a normal or increased level of insulin.[39] The disruption in the mechanism leads to metabolic abnormalities in PCOS. The occurrence of hyperinsulinemia in PCOS women was first reported by Burghen et al., in 1980.[40] It has been seen that PCOS women are prone to develop glucose intolerance and metabolic abnormalities followed by clinical manifestations such as ovulatory dysfunction and hyperandrogenism.[41,42] Surplus insulin acts as cogonadotropin either independently or along with LH on thecal cells and enhances the production of androgen.[43] Insulin acts directly on thecal cells with LH to enhance androgen production and by acting indirectly reduces the production of SHBG and IGF-1. Furthermore, elevated levels of androgen stimulate VAT to produce FFA which contributes to insulin resistance [Figure 3].[44]

- The merciless cycle among hyperinsulinemia and hyperandrogenemia for PCOS development. PCOS: Polycystic ovary syndrome.
Correlation between genetic, non-genetic, and hormonal factors
PCOS is a complex disorder, in which multiple factors are responsible for the pathogenesis of the disease. Factors such as genetic, environmental, and hormonal play a major role in the development of PCOS.[22] According to genetic studies, it has been seen that females with PCOS commonly have two main genetic alterations in the androgen metabolism process and insulin metabolism process, along with a high incidence of genetic polymorphism. Moreover, studies also suggest that environmental factors such as environmental toxins, obesity, and diet play a major role in the development of PCOS. It has been seen that the genetic alterations and environmental factors influence hormonal dysfunction which, in turn, leads to elevated levels of androgen, that is, hyperandrogenemia, and elevated levels of insulin, that is, hyperinsulinemia.[45,46] Furthermore, disturbance at hormone levels promotes multiple problems such as hirsutism, acne, menstrual disturbance, metabolic disorder, and cardiovascular disorder serves as major symptoms. The combined effect of all the factors contributes to the prognosis of PCOS [Figure 4].

- The interconnection between the risk factors responsible for the development of PCOS. PCOS: Polycystic ovary syndrome.
CONCLUSION
PCOS is an endocrine disorder accompanied by a metabolic disorder, mainly seen in women of reproductive age. Although the etiology is not very clear yet, the adverse effects of PCOS on ovulation and fertility are well known; therefore, the PCOS condition needs more attention. The condition becomes more adverse due to the major metabolic abnormalities which lead to multiple complications in females. The evidence available thus far suggests a strong correlation between the environmental, genetics, and hormonal factors in the pathology of PCOS. Understanding these factors will help in better management of this disorder. It has been concluded that women suffering from PCOS have two main genetic alterations seen in androgen metabolism and insulin metabolism with increased prevalence of gene polymorphism. Moreover, in recent times, studies are more focused on the molecular aspects such as genome-wide studies, for the identification of genetic factors, that is, candidate genes and pathways contributing to the complexity of this disorder and to analyze the exact mode of inheritance of this disease. Furthermore, studies on lifestyle factors have shown that PCOS women suffer hormonal imbalance, metabolic dysfunction, and reproductive abnormality. Moreover, environmental factors such as environmental toxins, diet, and obesity play a primary role in unveiling genetic susceptibility. Nowadays, treatment strategy needs to target the steriodogenesis genes which are mainly involved in the progression of PCOS which will pave the way for the development of personalized medicine, diagnosis, and treatment of PCOS, along with the hormonal parameters.
Acknowledgment
We acknowledge the Indian Council of Medical Research (5/10/15/CAR-SMVDU/2018-RBMCH) and DST (RP-93) for financial support.
AUTHORS’ CONTRIBUTIONS
M, R.K, and A.G planned the study. M wrote the manuscript. A.B, S.V, and G.C helped in the literature survey. R.S, N.T, A.B, G.R, and A.W critically edited the manuscript.
Declaration of patient consent
Patient consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Polycystic ovarian syndrome: An updated overview. Front Physiol. 2016;7:124.
- [CrossRef] [PubMed] [Google Scholar]
- Fundamental concepts and novel aspects of polycystic ovarian syndrome: Expert consensus resolutions. Front Endocrinol (Lausanne). 2020;11:516.
- [CrossRef] [PubMed] [Google Scholar]
- The correlation between hormonal disturbance in PCOS women and serum level of Kisspeptin. Int J Endocrinol. 2020;2020:6237141.
- [CrossRef] [PubMed] [Google Scholar]
- Infertility management in women with polycystic ovary syndrome: A review. Porto Biomed J. 2021;6:e116.
- [CrossRef] [PubMed] [Google Scholar]
- Cross-sectional study on the knowledge and prevalence of PCOS at a multiethnic university. Prog Prev Med. 2020;5:e0028.
- [CrossRef] [Google Scholar]
- The prevalence of polycystic ovarian syndrome in Chinese women: A meta-analysis. Ann Palliat Med. 2021;10:74-87.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and clinical features of polycystic ovary syndrome in Thai adolescents. . 2021;36:172-8.
- [Google Scholar]
- The prevalence of polycystic ovary syndrome: A brief systematic review. J Hum Reprod Sci. 2020;13:261-71.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of clinical manifestations, health risks, and quality of life among women with polycystic ovary syndrome. . 2019;14:e0223329.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology, pathogenesis, genetics and management of polycystic ovary syndrome in India. . 2019;150:333.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr Rev. 2009;30:293-342.
- [CrossRef] [PubMed] [Google Scholar]
- Environmental impacts on reproductive health and fertility. Cambridge, United Kingdom: Cambridge University Press; 2010. p. :1-21.
- [CrossRef] [Google Scholar]
- Potential adverse effects of female and male obesity on fertility: A narrative review. Int J Endocrinol Metab. 2020;18:e101776.
- [CrossRef] [Google Scholar]
- Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23:324-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril. 2001;75:53-8.
- [CrossRef] [Google Scholar]
- Instability of the human genome: Mutation and dna repair in human molecular genetics. Vol Vol. 2. New York: John Wiley and Sons Inc.; 1999. p. :209-17.
- [Google Scholar]
- Association of the steroid synthesis gene CYP11a with polycystic ovary syndrome and hyperandrogenism. Hum Mol Genet. 1997;6:397-402.
- [CrossRef] [PubMed] [Google Scholar]
- CYP11A1 microsatellite (tttta) n polymorphism in PCOS women from South India. J Assist Reprod Genet. 2014;31:857-63.
- [CrossRef] [PubMed] [Google Scholar]
- Association of the CAG repeat polymorphisms in androgen receptor gene with polycystic ovary syndrome: A systemic review and meta-analysis. . 2013;524:161-7.
- [CrossRef] [PubMed] [Google Scholar]
- The role of heterozygosity for CYP21 in the polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2000;13:1315-7.
- [Google Scholar]
- The association between androgen receptor gene CAG polymorphism and polycystic ovary syndrome: A case-control study and meta-analysis. J Assist Reprod Genet. 2014;31:1211-9.
- [CrossRef] [PubMed] [Google Scholar]
- Polycystic ovaries and premature male pattern baldness are associated with one allele of the steroid metabolism gene CYP17. Hum Mol Genet. 1994;3:1873-6.
- [CrossRef] [PubMed] [Google Scholar]
- CYP11A1 and CYP17 promoter polymorphisms associate with hyperandrogenemia in polycystic ovary syndrome. Fertil Steril. 2009;92(2):653-9.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic basis of polycystic ovary syndrome (PCOS): Current perspectives. Appl Clin Genet. 2019;12:249-60.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic variants associated with hyperandrogenemia in PCOS pathophysiology. Genet Res Int. 2018;2018:7624932.
- [CrossRef] [PubMed] [Google Scholar]
- Polycystic ovary syndrome: Role of aromatase gene variants in South Indian women. Int J Pharm Bio Sci. 2015;6:B1283-96.
- [Google Scholar]
- The role of androgen receptor activity mediated by the CAG repeat polymorphism in the pathogenesis of PCOS. J Med Life. 2013;6:18.
- [Google Scholar]
- The synergistic effect of sex hormone-binding globulin and aromatase genes on polycystic ovary syndrome phenotype. Eur J Endocrinol. 2008;158:861-6.
- [CrossRef] [PubMed] [Google Scholar]
- Localization of the human sex hormone-binding globulin gene (SHBG) to the short arm of chromosome 17 (17p12----p13) Cytogenet Cell Genet. 1990;54:65-7.
- [CrossRef] [PubMed] [Google Scholar]
- Transcription factors different from the estrogen receptor stimulate in vitro transcription from promoters containing estrogen response elements. Mol Cell Endocrinol. 1990;69:167-78.
- [CrossRef] [Google Scholar]
- A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:E48-56.
- [CrossRef] [PubMed] [Google Scholar]
- Sex hormone-binding globulin genetic variation: Associations with Type 2 diabetes mellitus and polycystic ovary syndrome. Minerva Endocrinol. 2010;35:271-80.
- [Google Scholar]
- Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: A review. Egyptian J Med Hum Genet. 2019;20:25.
- [CrossRef] [Google Scholar]
- The pathogenesis of polycystic ovary syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. . 2016;37:467-520.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of lifestyle intervention on the reproductive endocrine profile in women with polycystic ovarian syndrome: A systematic review and meta-analysis. Endocr Connect. 2014;3:36-46.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin Reprod Med. 2007;25:352-9.
- [CrossRef] [PubMed] [Google Scholar]
- Insulin-lowering agents in the management of polycystic ovary syndrome. Endocr Rev. 2003;24:633-67.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980;50:113-6.
- [CrossRef] [PubMed] [Google Scholar]
- Impaired glucose tolerance, Type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2010;16:347-63.
- [CrossRef] [PubMed] [Google Scholar]
- Androgens and insulin-two key players in polycystic ovary syndrome. Gynakol Geburtshilfliche Rundsch. 2008;48:9-15.
- [CrossRef] [PubMed] [Google Scholar]
- Metformin in the treatment of infertility in polycystic ovarian syndrome: An alternative perspective. Fertil Steril. 2008;90:14-6.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic markers of polycystic ovary syndrome: Emphasis on insulin resistance. Int J Med Genet. 2014;2014:478972.
- [CrossRef] [Google Scholar]
- Polycystic ovary syndrome and insulin-resistant hyperinsulinemia. J Am Acad Dermatol. 2001;45:S95-104.
- [CrossRef] [PubMed] [Google Scholar]
- The role of hyperinsulinemia in the pathogenesis of ovarian hyperandrogenism. Fertil Steril. 1988;50:197-212.
- [CrossRef] [Google Scholar]






