Translate this page into:
Progesterone supplemented uterine epithelial cell co-culture improves in vitro quality embryo production in buffalo

*Corresponding author: Vikash Chandra, Principal Scientist, Division of Physiology and Climatology, ICAR-Indian Veterinary Research Institute, Bareilly, Uttar Pradesh, India. vikashvet15@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pandey S, Bharti MK, Bhat IA, Chandra V, Taru Sharma G. Progesterone supplemented uterine epithelial cell co-culture improves in vitro quality embryo production in buffalo. J Reprod Healthc Med 2024;5:10.
Abstract
Objectives:
The present study was conducted to study the effect of progesterone and uterine luminal epithelial cells monolayer on blastocyst development and hatching rate.
Material and Methods:
The isolation, culture, and characterization of slaughterhouse-derived uterine epithelial cells were done using standard protocol. The steroid hormones estrogen and progesterone were supplemented in embryo developmental media (EDM), and day 04 embryos were cultured in different groups as progesterone supplementation (T1), co-cultured with epithelial cell monolayer (T2), co-cultured with progesterone supplemented epithelial cell monolayer (T3), or without any treatment (control). Finally, the effect of different treatments was analyzed in terms of blastocyst and hatching rate.
Results:
The isolated epithelial cells depicted compact cuboidal or columnar morphology at the confluence. Immunocytochemical localization and polymerase chain reaction study revealed positive expression of cytokeratin and absence of vimentin. Significantly higher blastocyst and hatching rates were noted in the T3 group, followed by T2, T1, and control groups.
Conclusion:
The present study revealed improved in vitro embryo production after co-culturing embryos with progesterone-supplemented uterine epithelial cells in buffalo.
Keywords
Progesterone
Epithelial cells
Buffalo
Embryo
INTRODUCTION
Buffalo constitutes 19.89% of total livestock and contributes about 53% of total milk production in India. To meet out demand for buffalo milk and meat for the ever-increasing human population, faster propagation of superior germplasm of buffalo is required.[1] The progesterone concentration has a vital role in the establishment and maintenance of pregnancy, conceptus elongation,[2] production of interferon-tau,[3] and in bovine and ovine species.[4] In bovine, most of the pregnancy loss occurs during days 8–16 of pregnancy[5], mainly due to lower circulating progesterone (P4), which may be through the modulation of endometrial gene expression.[6] Blastocyst development and elongation inhibit luteolysis by the secretion of interferon tau (IFNτ), which is crucial for pregnancy recognition signaling.[5] The histotrophs are thought to be a major factor supporting the growth of pre-implantation blastocysts and elongating conceptus.[7] It can be hypothesized that culturing early morula stage embryos in progesterone-primed luminal epithelial cell monolayer may improve blastocyst production and hatching rate.
The endometrium consists of epithelial (luminal and glandular) and stromal cells. In vitro cell culture of either single or mixed cells has been successively performed on mice, guinea pigs, pigs, humans, cows, and sheep.[8] The advantages of these cell cultures are to study the early phases of implantation and to create an environment to study the functions of uterine cells. In vitro culture of endometrial cells also enables us to understand the action of specific cofactors by eliminating or adding them in a step-wise manner.[9] Hence, the present study was planned with the objective to study the effect of progesterone-supplemented uterine luminal epithelial cells monolayer on in vitro embryonic development in buffalo.
MATERIAL AND METHODS
The procurement of materials was done from reputed firms such as plastic wares from Nunc (Denmark), 0.22 µm membrane filters from Millipore (Molsheim, France), and chemicals and culture media from Sigma Chemicals Co., (St. Louis, MO, USA). Specifications and descriptions of the chemicals, molecular reagents, and antibodies are given at suitable places in the text.
Isolation of uterine epithelial cells
The isolation, culture, and characterization of uterine epithelial cells (UECs) were done using 0.25% trypsinethylenediaminetetraacetic acid (EDTA).[10] Briefly, early luteal phase bubaline uteri were procured from the slaughterhouse, washed, and flushed twice with 15–20 mL of warm phosphate-buffered saline (70011-044, Gibco Life Technologies) supplemented with 0.1% bovine serum albumin (BSA) (A3311, Sigma) and 100 µg/mL gentamycin. Both uterine horns were flushed with 0.25% trypsin-EDTA (T4799, Sigma; E6758) and kept at 38.5°C, 70–100 rpm for 60 min in a shaker incubator (Matrix Eco Solution, India). Then, uterine contents were aspirated using a sterile syringe, centrifuged at 250 g for 10 min, and resuspended in high-glucose Dulbecco’s Modified Eagle Medium (D5796, Sigma) supplemented with 10% fetal bovine serum (FBS) (F2442, Sigma) and gentamycin sulfate solution @50 µg/mL. Finally, the cell suspension was strained with a 70 µm cell strainer (352350, BD Falcon™, USA), and the cell viability test was assessed using 0.4% trypan blue dye (T8154, Sigma). The isolated cells were cultured in 24 well culture plates (142475, Thermo Scientific) @ 5 × 104 cells/cm2. The culture medium was first replaced at 24 hours and then on alternate days till 70–80% cell confluence.
Characterization of uterine epithelial cells
The UECs characterization was based on the cell attachment rate, its phenotype, immune-localization, and mRNA expression of cell-specific markers as per the protocol described by Pandey et al.[10]
The attachment of the cell was observed at every 6 h interval under an inverted microscope (Olympus, Japan). Phenotypic characterization of primary culture was done by observing the shape, compactness, and growth characteristics of attached cells. The immunocytochemistry (ICC) and polymerase chain reaction (PCR) assay were conducted for the localization of epithelial cell-specific markers. A minimum of three trials were conducted for each experiment, and the mean value of each triplicate trial was taken into consideration for the analysis of the results.
The primary uterine epithelial cell culture was used for the ICC study. The cells at about 60 per cent confluence were processed for localization of cytokeratin and vimentin using primary antibody against cytokeratin (Santa Cruz; sc-32328), a positive marker of epithelial cells and vimentin (Novus Biologicals; NBP1-31327), and a negative marker of epithelial cells at a dilution of 1:100 dilution for each. Further, cells were incubated with a secondary antibody having a dilution rate of 1: 200 at 37°C for 2 h in a dark environment. The secondary antibodies used are Texas Red (sc-2785) conjugated donkey anti-mouse and phycoerythrin (sc-3739) conjugated goat anti-rabbit antibody. The cells were then counterstained with 4’, 6-diamidino-2-phenylindole (sc-3598). The exclusion of primary antibodies was ensured in negative control. Images were taken in the fluorescent microscope (IX 71, Olympus, Shinjuku, Tokyo, Japan).
Total RNA was extracted from primary uterine epithelial cell culture by Trizol (90305; Ambion, Life Technologies, USA) method and PCR was done following the standard protocol. The quality and integrity of the RNA were determined using 1% agarose gel electrophoresis, and the concentration and purity were checked by the nanodrop spectrophotometer measuring absorbance at 260/280 against nuclease-free water as a blank. Total RNA with OD260/280 between 1.8 and 2.0 were processed for cDNA synthesis using the Verso cDNA synthesis kit (AB- 1453/B; Thermo Scientific, USA). As a template, 1 μg total RNA was used for cDNA synthesis. The PCR amplification was conducted using Platinum PCR supermix (11306-016; Life Technologies, USA) in a thermal cycler (XP Cycler, Bioer). Briefly, the initial denaturation was done at 94°C for 2 min, then 35 amplification cycles of denaturation at 94°C for 30 s, followed by annealing at a primer-specific annealing temperature for 30 s and the final extension at 72°C for 30 s. Both negative reverse transcriptase control and negative template control were set for each set of primers. The details of the genes studied are provided in Table 1. The agarose gel electrophoresis (1.8%) was used to confirm the amplification of a specific reverse transcription-PCR product and the results were noted on a gel documentation system (Syngene G: BOX, USA).
| Gene | Primer sequence (5’-3’) | Annealing temperature (°C) | Amplicon size (bp) | Accession no. |
|---|---|---|---|---|
| Cytokeratin | F-CCCCCAGGTCCTTCAGCAGCC | 64 | 147 | XM_006046605.2 |
| R-GGGCCCCACCGTAGCTTCCAG | ||||
| Vimentin | F-CCGACGCCATCAACACCGAGT | 60 | 163 | XM_006717500.2 |
| R- TTGCCCTGGCCCTTGAGCTG | ||||
| GAPDH | F-GCGATACTCACTCTTCTACTTTCGA | 58 | 82 | U85042.1 |
| R- TCGTACCAGGAAATGAGCTTGAC |
bp: Base pair, GAPDH: Glyceraldehyde 3 phosphate dehydrogenase
Exposure of epithelial cells to steroid hormone
To mimic the natural internal milieu of the uterus, steroid hormones were supplemented to primary uterine epithelial cells. The 17β-estradiol was supplemented at concentrations of 10 pg/mL for 24 h. Afterwards, the progesterone and estradiol were added in cell culture for 6 days at concentrations of 3.14 ng/mL and 05 pg/mL, respectively.
In vitro embryo culture
Bubaline embryos were produced in vitro as per the protocol described by Bhardwaj et al.[11] and Pandey et al.[12] with few modifications. In brief, bubaline ovaries were procured from a local abattoir, thoroughly washed in normal saline solution, and antral follicles were aspirated using a 5 mL sterile disposable syringe. The cumulus-oocyte complexes (COCs) were graded based on their morphology and ooplasm homogeneity to select grade A and B COCs for further culture.[13] In vitro maturation of procured immature COCs was done in TCM 199 maturation media (HEPES modified) containing 0.5µg/mL follicle-stimulating hormone, 5 µg/mL luteinizing hormone, 0.25 mM sodium pyruvate, 0.68 mM L-glutamine, 1 µg/mL estradiol-17β, 3 mg/mL BSA, 10% FBS, 10 µg/mL gentamicin for 24 h at 5% carbon dioxide (CO2), and 38.5°C temperature in incubator with maximum relative humidity. Cryopreserved-thawed buffalo semen was used for in vitro fertilization (IVF) and processed by swim-up technique. The FerTALP (Tyrode-albumin-lactatepyruvate) media was used for IVF, which contains 20 µg/mL heparin, 0.2 mM sodium pyruvate, and 6 mg/mL BSA. The 10–15 in vitro matured washed COCs were coincubated in the 50µl processed semen droplets at 38.5°C and 5% CO2 for 18 h. After co-incubation, presumptive zygotes (10–15) were transferred in a 50 µL drop of EDM containing 1% essential and non-essential amino acids, 3 mg/mL BSA (fatty acid-free), 0.25 mM sodium pyruvate, 50 µg/mL gentamycin, and 0.68mM L-glutamine. Then, embryos were cultured with EDM alone as a control, EDM supplemented with 3.14 ng/ml P4, epithelial cell monolayer, and steroid-treated epithelial cell monolayer.[12] The media was changed on alternate days till blastocyst development or hatching. The cleavage rate was recorded as the number of cleaved embryos out of a number of presumptive zygotes cultured. The blastocyst rate (BR) was calculated as the total blastocysts formed out of the total cleaved embryos. The hatching rate was noted as a total hatched blastocysts out of a total blastocysts formed.
Co-culture of the embryo and uterine epithelial cells
Day 04 embryos or morula were co-cultured with cultured luminal epithelial cells (0 ng/mL progesterone), 3.14 ng/mL progesterone, and steroid-treated luminal epithelial cells monolayer (3.14 ng/mL progesterone) till day 07–09 post-IVF. The cleavage rate was recorded as the number of cleaved embryos out of a number of presumptive zygotes cultured. The BR was calculated as the total blastocysts formed out of the total cleaved embryos. The hatching rate was noted as total hatched blastocysts out cleavage, blastocyst development, and hatching in culture without progesterone supplementation and any epithelial cell monolayer bedding was taken as control.
Statistical analysis
The statistical analysis was done using SAS 9.2 software (SAS Institute Inc., Cary, NC, USA). Values are noted as the mean ± standard error of the mean. The values with P < 0.05 were considered significant.
RESULTS
The uterine epithelial cells were successfully isolated and characterized. The cellular clumps or explants were observed in the culture plate within 24 hours of seeding. Afterwards, these cells migrated out and formed epithelial cell colonies, which reached confluence on days 7–8. The uterine epithelial cells revealed compact cuboidal or columnar morphology on confluence but with a number of passages, cells start losing their morphology as well as compactness [Figure 1a-d].
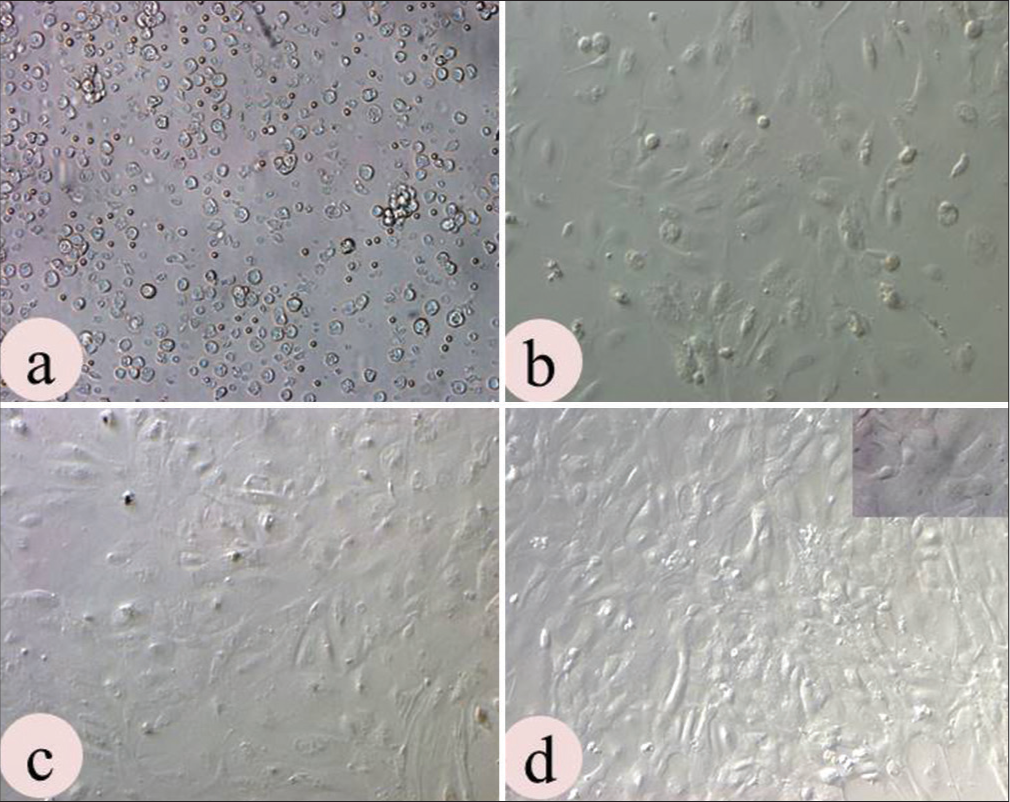
- Culture characteristics of uterine epithelial cells (UECs): (a) Heterogeneous population observed at cell seeding; (b) Assumed characteristic shape on day 8 and (c) Became confluent on day 14; (d) UECs achieved characteristics compactness on P3 culture. (Magn. 10×).
The ICC results indicated the presence of cytokeratin with a bright fluorescence and the absence of vimentin with no fluorescence [Figure 2].
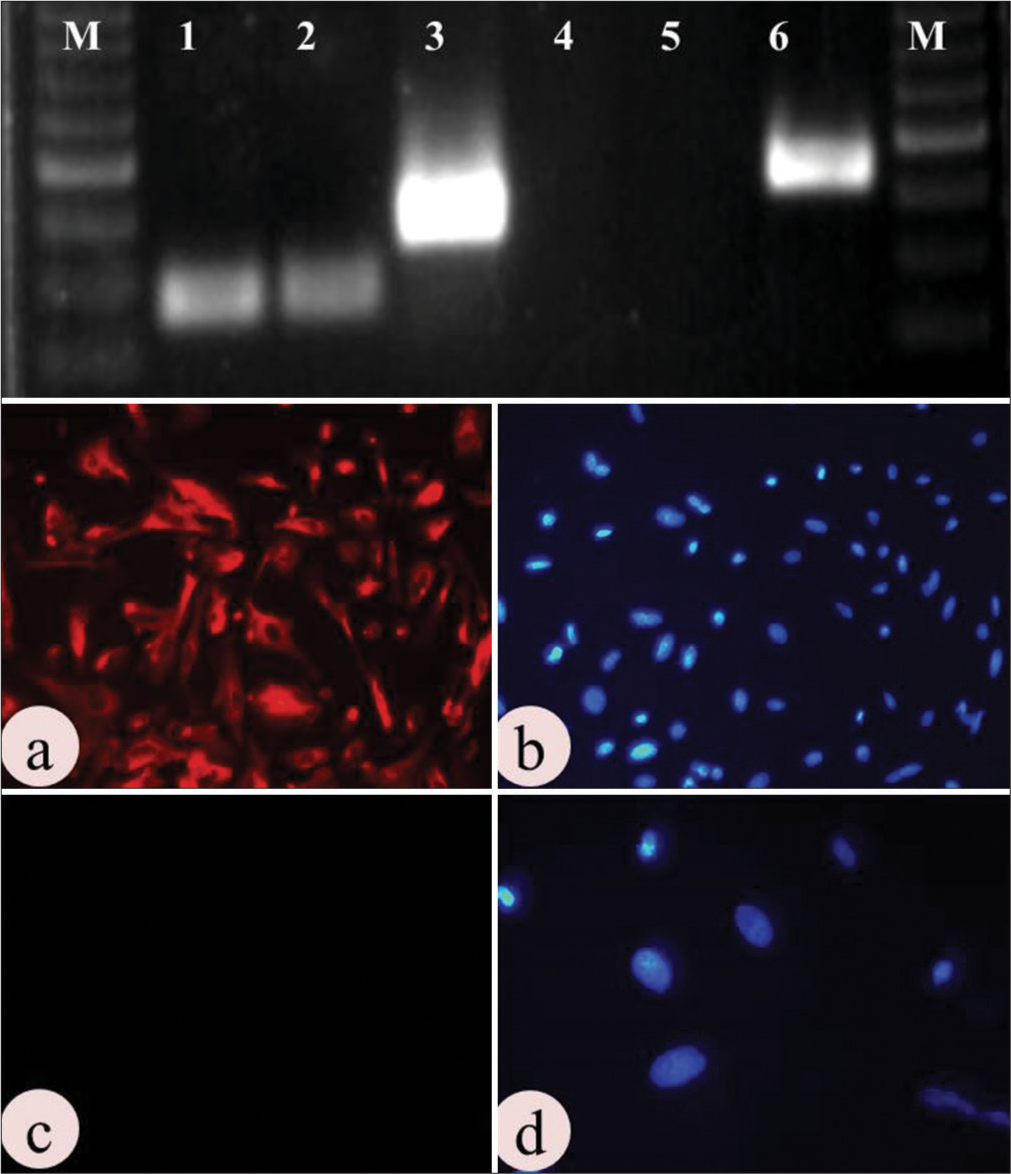
- Molecular characterization of uterine epithelial cells: Upper panel shows Agarose gel electrophoresis photograph of epithelial and stromal cell positive and negative markers: M-50 bp marker, 1- GAPDH (82 bp) in epithelial cell, 2- GAPDH (82 bp) in stromal cell, 3- Cytokeratin (147bp) in epithelial cell, 4- Cytokeratin (147bp) in stromal cell, 5- Vimentin (163 bp) in epithelial cell, 6- Vimentin (163 bp) in stromal cell. Lower panel shows immunolocalization of: (a-b) Cytokeratin (positive), and (c-d) Vimentin (Negative). (Mag. 10×).
A single dark, bright band of cytokeratin was observed at 147 bp position in agarose gel electrophoresis, whereas no band was noted in the vimentin lane (163 bp) of epithelial cells, whereas vice versa was noted down for stromal cells. GAPDH of 82 bp was kept as an internal control for both the cell types, which also showed a bright band in the gel picture [Figure 2].
These successfully isolated and well-characterized epithelial cells were then used in further experimental trials. Embryonic development data is represented in Table 2. The study depicted no (P < 0.05) difference in the cleavage rate of control and treatment groups (T1, T2, and T3). However, a significantly (P < 0.05) higher BR was observed in the T3 group than in the T2 group (34.69 and 28.44, respectively). Further, the control and T1 (20.54 and 25, respectively) groups did not reveal any significant (P > 0.05) difference in BR, but it was significantly (P < 0.05) lower than the T3 and T4 group. The graphical representation of BR in control and treatment groups is shown in Figure 3.
| Group | COCs (n) | Cleavage rate n(%) | Blastocyst rate n(%) | Hatching rate n(%) |
|---|---|---|---|---|
| Control | 338 | 224 (66.27) | 46 (20.54)a | 6 (2.68)a |
| Treatment 1 (T1) | 302 | 200 (66.23) | 47 (23.5)a | 8 (8)ab |
| Treatment 2 (T2) | 324 | 218 (67.3) | 62 (28.44)b | 14 (6.42)bc |
| Treatment 3 (T3) | 294 | 196 (66.67) | 68 (34.69)c | 18 (9.18)c |
Values (mean percentage) in the column bearing different superscripts differ significantly (P<0.05). COCs: Cumulus oocyte complexes, a, b, c, ab and ac are superscripts showing significant difference.
A significantly (P < 0.05) higher hatching rate was noted in the T3 and T2 groups than in the control and T1 groups. Furthermore, better blastocyst development, along with a greater number of advance-stage blastocysts, was noted after progesterone supplementation in culture media. A graphical representation of the hatching rate has been presented in Figure 3. The pictorial representation of embryonic development in control and treatment groups is given in Figure 4.
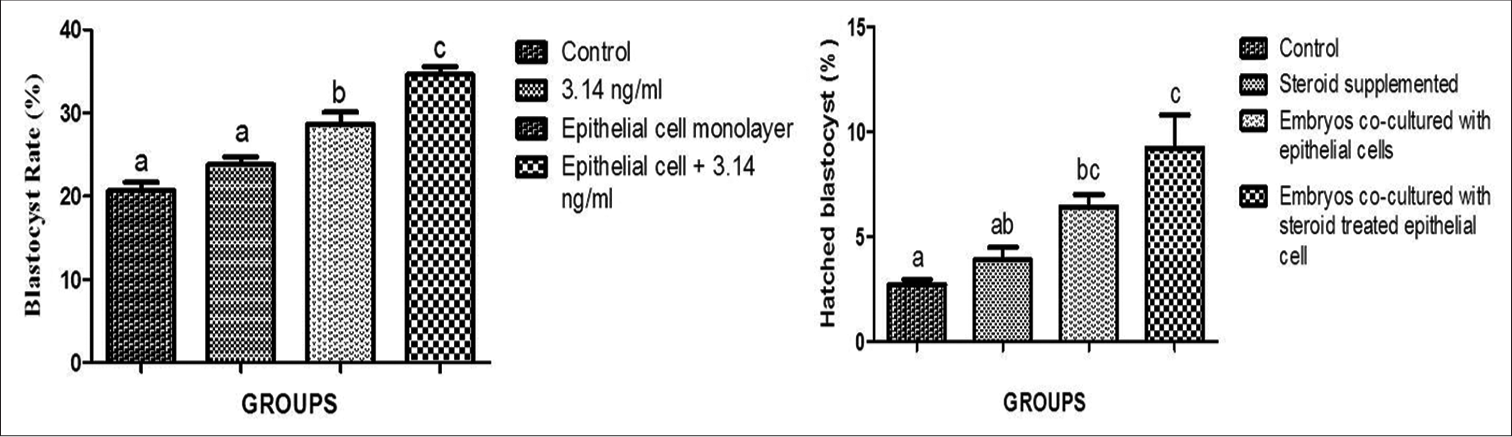
- Graphical representation blastocyst rate and hatching rate in different groups. 1st bar represents, control group which is without any supplementation, 2nd bar represents, Day 04 embryos co-cultured with 3.14 ng/ml progesterone hormone, 3rd bar represents, Day 04 embryos co-cultured with epithelial cell monolayer and 4th bar represents, Day 04 embryos co-cultured with progesterone treated epithelial cell monolayer. Bars bearing different superscripts differ significantly (P < 0.05).
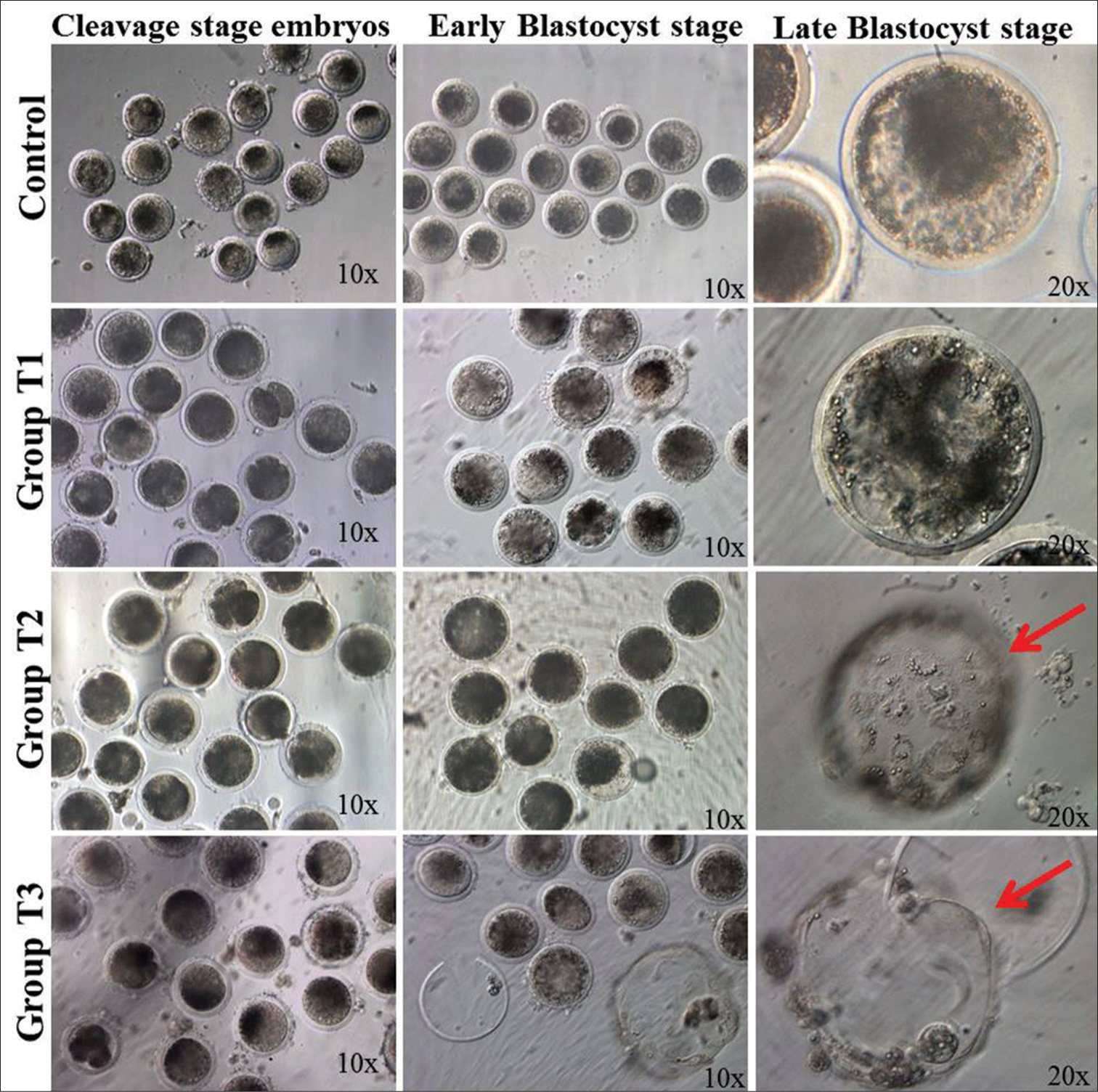
- Phase contrast photomicrographs of different stages of embryos in different treatment groups: Faster and better blastocysts were produced in the P4-supplemented epithelial cell monolayer co-culture system. The red arrow indicates hatched blastocysts in the T2 and T3 groups.
DISCUSSION
In the present study, the bubaline uterine epithelial cells were successfully isolated, cultured, characterized, and treated with steroid hormones. The present study determines the effect of progesterone hormone, uterine epithelial cell monolayer, and progesterone-primed uterine epithelial cell monolayer co-culture on blastocyst development and hatching rate in buffalo. The uterine epithelial cells are the first cells to interact with the implanting embryo. In vitro culture of these cells may provide a useful model to study uterine secretory activity, interactions with developing embryos, pregnancy recognition, and embryo implantation. In this study, the epithelial cells from abattoir-derived buffalo uterus were successfully isolated using enzymatic solution, trypsin– EDTA. Epithelial cells from uterine tissue have already been cultured in several species, including humans,[14] rats,[15] mice,[16] pigs,[17,18] cattle,[19] and buffalo[10,12,20] by different methods with varied purity of epithelial cells. The isolated epithelial cells during the present study provided an excellent pure cell monolayer and cultured in vitro successively. The isolated luminal epithelial cell revealed its characteristic properties, as reported by Pandey et al.[10,12]
The experiment was planned to determine the role of growth factors, cytokines, proteases, and other epithelial cell-secreted factors on the loss of zona pellucida during blastocyst hatching. During the present study, apart from the positive effect of epithelial cell monolayer on the hatching rate, an improved blastocyst development rate was also noted. However, a more pronounced effect was seen after steroid treatment of epithelial cells. Supplementation of progesterone alone did not improve the blastocyst development. These findings correlate with the fact of steroid hormone absorption by the mineral oil over the culture media drops.[21,22] Our results corroborate the findings of various other coworkers, where the supplementation of progesterone to culture media did not affect bovine blastocyst formation.[23] A direct relationship between increased progesterone concentration and embryo development was not depicted by Pereira et al. [24], whereas Carter et al.[25] noted a positive effect of elevated progesterone concentrations on post-hatching elongation but no effect on the recovery of blastocysts from single-ovulating animals. Similarly, the effect of progesterone concentration on BR after the endoscopic transfer of in vitro-produced zygotes has not been observed by Rings et al.[26] This signifies the fact that progesterone affects blastocyst development by advancing the endometrial transcriptome.[6] Contrary to these reports as well as our results, Ferguson et al.[27] depicted enhanced in vitro blastocyst development in bovines after progesterone supplementation in culture media. In another finding, the BR improved after co-culture with steroid-treated epithelial cells but it did not affect the hatching rate significantly.[28]
The understanding of complex interactions between an embryo and a dam demands an in vitro model which resembles the uterine environment. These models or cell cultures must be improved to support early embryonic development.[29] The beneficial effects of co-culture on embryo development rate may be due to the secretory embryotrophic factors of the epithelial cells,[30] reduction of oxygen tension,[31] and concentration of glucose.[32] These findings suggest that the effect of progesterone on the ability of the oocyte to form a blastocyst is dependent on the modulation uterine environment.
CONCLUSION
The present study concluded that the modulation of uterine epithelial cell secretomes by the progesterone hormone plays an important role in early blastocyst development in buffalo.
Acknowledgments
The authors are thankful to the Director of the institute for financial support for the study. Further, it is to state that the authors have no conflicts of interest to declare.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Animal biotechnology: Application and economic implications in developing countries. Rev Sci Tech. 2005;24:127-39.
- [CrossRef] [PubMed] [Google Scholar]
- Discovery of candidate genes and pathways in the endometrium regulating ovine blastocyst growth and conceptus elongation. Physiol Genomics. 2009;39:85-99.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of time of progesterone supplementation on embryo development and interferon-tau production in the cow. Vet J. 2006;171:500-3.
- [CrossRef] [PubMed] [Google Scholar]
- Associations between milk progesterone concentration on different days and with embryo survival during the early luteal phase in dairy cows. Theriogenology. 2006;65:1435-41.
- [CrossRef] [PubMed] [Google Scholar]
- Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol Reprod. 2009;81:784-94.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction. 2002;124:289-300.
- [CrossRef] [PubMed] [Google Scholar]
- Why does the fallopian tube fail in ectopic pregnancy? The role of activins, inducible nitric oxide synthase and MUC1 in ectopic implantation. Fertil Steril. 2012;97:1115-23.
- [CrossRef] [PubMed] [Google Scholar]
- Isolation and characterization of endometrial luminal epithelial and stromal cells in vitro. Sokoto J Vet Sci. 2014;12:1-8.
- [CrossRef] [Google Scholar]
- Expression profile of adhesion molecules in blastocyst vis-a-vis uterine epithelial cells. Theriogenology. 2021;170:36-45.
- [CrossRef] [PubMed] [Google Scholar]
- GREM1, EGFR, and HAS2; the oocyte competence markers for improved buffalo embryo production in vitro. Theriogenology. 2016;86:2004-11.
- [CrossRef] [PubMed] [Google Scholar]
- Progesterone modulates adhesion molecules in uterine epithelial cells and in vitro embryo production in buffalo. Reprod Domest Anim. 2020;55:833-43.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of roscovitine on developmental competence of small follicle-derived buffalo oocytes. Indian J Med Res. 2018;148:S140-50.
- [Google Scholar]
- Coculturing human endometrial epithelial cells and stromal fibroblasts alters cell-specific gene expression and cytokine production. Fertil Steril. 2013;100:1132-43.
- [CrossRef] [PubMed] [Google Scholar]
- Differential response of individual uterine cell types from immature rats treated with estradiol. Endocrinology. 1980;106:1634-49.
- [CrossRef] [PubMed] [Google Scholar]
- A model for implantation: Coculture of blastocysts and uterine endometrium in mice. Biol Reprod. 2005;72:556-61.
- [CrossRef] [PubMed] [Google Scholar]
- Establishment and characterization of female reproductive tract epithelial cell culture. J Microsc Ultrastruct. 2017;5:105-10.
- [CrossRef] [PubMed] [Google Scholar]
- Prostaglandin formation by the sheep embryo and endometrium as an indication of maternal recognition of pregnancy. Biol Reprod. 1981;25:56-64.
- [CrossRef] [PubMed] [Google Scholar]
- Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide. Biol Reprod. 2012;87:135.
- [CrossRef] [PubMed] [Google Scholar]
- Freezing of endometrial epithelial cells of buffalo cultured in vitro. Indian J Anim Sci. 2013;83:7.
- [Google Scholar]
- Delay of nuclear maturation and reduction in developmental competence of pig oocytes after mineral oil overlay of in vitro maturation media. Reproduction. 2002;124:557-64.
- [CrossRef] [PubMed] [Google Scholar]
- 17Beta-estradiol and progesterone improve in-vitro cytoplasmic maturation of oocytes from unstimulated prepubertal and adult rhesus monkeys. Hum Reprod. 2003;18:2137-44.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of supplemental progesterone on the development, metabolism and blastocyst cell number of bovine embryos produced in vitro. Reprod Fertil Dev. 2011;23:311-8.
- [CrossRef] [PubMed] [Google Scholar]
- Embryos and culture cells: A model for studying the effect of progesterone. Anim Reprod Sci. 2009;111:31-40.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of increasing progesterone concentration from day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reprod Fertil Dev. 2008;20:368-75.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of elevated circulating progesterone concentration on development of in vitro produced bovine zygotes in vivo. Reprod Fertil Dev. 2008;20:147-8.
- [CrossRef] [Google Scholar]
- Progesterone enhances in vitro development of bovine embryos. Theriogenology. 2012;77:108-14.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of steroid treatment of endometrial cells on blastocyst development during co-culture. Theriogenology. 1998;49:1021-30.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of a novel co-culture system on development, metabolism and gene expression of bovine embryos produced in vitro. Reproduction. 2002;124:543-56.
- [CrossRef] [PubMed] [Google Scholar]
- Early embryonic signals: Embryo maternal interactions before implantation. Anim Reprod Sci. 1992;28:269-76.
- [CrossRef] [Google Scholar]
- Factors affecting the in vitro development to blastocysts of bovine oocytes matured and fertilized in vitro. J Reprod Fertil. 1991;92:125-31.
- [CrossRef] [PubMed] [Google Scholar]
- Culture of preimplantation embryos: Facts and artifacts. Hum Reprod Update. 1995;1:91-148.
- [CrossRef] [PubMed] [Google Scholar]






