Translate this page into:
Nanotechnology advances in treatment of reproductive diseases: From bench to bedside

*Corresponding author: Pankaj Vinodrao Dixit, Department of Pharmacology, Sun Pharmaceutical Industries Limited, Vadodara, Gujarat, India. pankaj-dixit@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dixit PV, Mishra DK. Nanotechnology advances in treatment of reproductive diseases: From bench to bedside. J Reprod Healthc Med. 2025;6:1. doi: 10.25259/JRHM_28_2024
Abstract
Nanotechnology is the manipulation of matter at nanoscale, typically involving structures with dimensions between 1 and 100 nm. At this tiny scale, the properties of materials can differ significantly from their bulk counterparts due to quantum mechanical effects and increased surface area. Developing targeted drug delivery systems and novel drug delivery tools is mainly addressed by the field of nanomedicine. Nanomedicine has emerged as a transformative tool in the diagnosis and treatment of reproductive diseases, offering innovative solutions from early detection to advanced therapeutic strategies. Reproductive diseases, such as infertility, endometriosis, ovarian and testicular cancers, and polycystic ovary syndrome, present significant challenges in medical practice due to their complex etiology and treatment limitations. Traditional methods often involve invasive treatments with substantial side effects and limited success rates. By utilizing nanoparticles, nanosensors, and advanced imaging techniques, nanotechnology enhances early disease detection, improves diagnostic precision, and allows for targeted drug delivery, reducing systemic side effects. This review explores cutting-edge nanotechnology applications, such as polymer-based nanoparticles, liposomes, micelles, dendrimers, and gene therapy delivery systems in reproductive health. It also discusses the safety concerns, ethical considerations, and evolving regulatory frameworks that must accompany the integration of nanomedicine in reproductive treatments. The findings highlight nanotechnology’s potential to revolutionize reproductive healthcare, offering personalized and more effective treatment modalities from bench to bedside.
Keywords
Biomedical imaging
Gene therapy
Nanocarriers
Nanoparticles
Nanosensors
Targeted therapy
Translational medicine
INTRODUCTION
Reproductive diseases encompass a wide range of conditions that affect the reproductive system in both men and women, including infertility, endometriosis, ovarian and testicular cancers, polycystic ovary syndrome (PCOS), and sexually transmitted infections (STIs).[1,2] These conditions can lead to significant physical, emotional, and social impacts, affecting overall quality of life. Traditional treatments often involve invasive procedures, hormonal therapies, or long-term medication, which may come with considerable side effects and variable success rates.[3]
Nanotechnology has emerged as a transformative force in the field of medicine, offering novel solutions for the diagnosis, treatment, and prevention of various diseases, including those related to reproductive health.[4] By leveraging the unique properties of nanoscale materials, such as their high surface area-to-volume ratio and ability to interact with biological systems at the molecular level, nanotechnology enables precise drug delivery, enhanced imaging techniques, and innovative therapeutic approaches. In the context of reproductive diseases, nanotechnology holds the potential to revolutionize how these conditions are detected and managed, paving the way for more effective and personalized treatments.[5,6]
This article aims to explore the latest advancements in nanotechnology as applied to the diagnosis and treatment of reproductive diseases. It will provide a comprehensive overview of how nanotechnology is being integrated into reproductive medicine, from basic research at the bench to clinical applications at the bedside.[6] The review will cover various nanotechnology-based diagnostics, therapeutic strategies, and their application in specific reproductive disorders while also addressing the associated safety, ethical, and regulatory considerations.[7,8] By highlighting current developments and future trends, the review seeks to provide readers with an in-depth understanding of the potential of nanotechnology to transform reproductive health care.[9]
NANOPARTICLES IN EARLY DETECTION AND SCREENING
Nanoparticles have revolutionized the early detection and screening of reproductive diseases by offering highly sensitive and specific tools for identifying disease markers at the molecular level.[10] Due to their small size and customizable surface properties, nanoparticles can be engineered to target specific cells or proteins associated with conditions such as ovarian cancer, endometriosis, or STIs [Table 1].[11,12] These targeted nanoparticles enhance the accuracy of diagnostic tests, enabling the detection of diseases at much earlier stages than traditional methods, thereby improving patient outcomes through timely intervention.[13]
| Reproductive disease | Nanoparticle type | Targeted biomarker/cell | Diagnostic advantage | References |
|---|---|---|---|---|
| Ovarian cancer | Gold nanoparticles | CA125, HE4 | Enhanced sensitivity for early detection | Loo et al.,[10] Muthu and Feng,[11] Lu et al.,[12] Zang et al.[13] |
| Endometriosis | Polymer-based nanoparticles | Endometrial cells | Improved localization of endometriotic lesions | Muthu and Feng,[11] Lu et al.[12] |
| Sexually transmitted infections (STIs) | Quantum dots | Bacterial/Viral pathogens | Rapid detection with high specificity | Holzinger et al.,[14] Dykman and Lhlebtsov,[15] Rosi and Mirkin,[16] Grainger and El-Sayed[17] |
| Polycystic ovary syndrome (PCOS) | Magnetic nanoparticles | Insulin receptors, Androgen | Early screening of hormonal imbalances | Loo et al.,[10] Dykman and Lhlebtsov[15] |
NANOSENSORS FOR BIOMARKER DETECTION
Nanosensors represent a significant breakthrough in the early detection and management of reproductive diseases, offering highly sensitive and specific diagnostic tools that are revolutionizing the landscape of reproductive health. These nanoscale devices are designed to detect extremely low concentrations of biomarkers – molecular indicators that signal the presence of a disease or abnormal physiological condition – in various bodily fluids such as blood, urine, and reproductive secretions. Biomarkers play a critical role in the diagnosis and monitoring of reproductive conditions such as infertility, PCOS, endometriosis, STIs, and reproductive cancers (e.g., ovarian and testicular cancers). However, traditional diagnostic methods often lack the sensitivity required to detect these biomarkers at early stages, when treatment interventions are most effective.
Nanosensors offer a solution to this challenge by enabling real-time, point-of-care diagnostics with a level of precision and sensitivity that far exceeds conventional methods. These sensors are typically engineered from nanomaterials, including carbon nanotubes, quantum dots, gold nanoparticles, and nanowires, which are capable of binding to specific biomarkers due to their high surface area and functionalization capabilities. This interaction between the nanosensor and the biomarker leads to detectable changes in the sensor’s properties – such as electrical conductivity, fluorescence, or colorimetry – that can be measured and quantified to provide rapid diagnostic results.[14]
One of the primary advantages of nanosensors is their ability to be integrated into point-of-care diagnostic devices. This allows for on-the-spot, non-invasive testing that delivers immediate results, bypassing the need for complex laboratory procedures. This capability is particularly beneficial in reproductive health, where early and accurate diagnosis is essential for effective treatment planning.[15] For example, in cases of infertility or PCOS, nanosensors can detect hormonal imbalances or metabolic biomarkers in bodily fluids, enabling clinicians to diagnose the condition more rapidly and tailor treatments to the individual patient’s needs. Similarly, in reproductive cancers, nanosensors can detect specific tumor markers at much earlier stages than traditional imaging or biopsy techniques, allowing for early intervention and improving the chances of successful treatment outcomes.
Nanosensors also offer potential benefits in the continuous monitoring of reproductive diseases. Patients with chronic conditions, such as endometriosis or PCOS, could benefit from wearable or implantable nanosensor devices that provide real-time monitoring of biomarker levels. This would allow for more dynamic management of the disease, with treatments being adjusted based on real-time data rather than relying solely on periodic clinical visits and retrospective assessments. The ability to monitor biomarkers over time also enables the detection of disease progression or recurrence, such as in the case of ovarian cancer survivors, where early detection of relapse is critical for timely intervention.[16]
In addition to their diagnostic applications, nanosensors are paving the way for more personalized treatment strategies in reproductive health. By identifying specific biomarkers unique to an individual’s condition, nanosensors can guide the selection of targeted therapies, reducing the trial-and-error approach often associated with reproductive treatments. For instance, the detection of certain biomarkers in patients with reproductive cancers could inform the choice of chemotherapeutic agents or targeted therapies, improving treatment efficacy while minimizing adverse effects.[17]
Despite their promise, the development and implementation of nanosensors in clinical settings come with challenges. Key considerations include ensuring the biocompatibility of nanosensors, avoiding potential toxicity, and addressing the scalability and cost-effectiveness of these devices for widespread use in reproductive health diagnostics. In addition, regulatory frameworks must evolve to ensure that nanosensor-based diagnostics meet stringent safety and efficacy standards before they can be integrated into routine clinical practice.
IMAGING TECHNIQUES ENHANCED BY NANOTECHNOLOGY
Nanotechnology is revolutionizing the field of reproductive health by significantly improving both diagnostic and therapeutic approaches. Innovations such as nanosensors, nano-enhanced imaging techniques, and nanocarriers for drug delivery are providing more precise, effective, and personalized care for patients. From enhancing the sensitivity of diagnostic tools to enabling targeted therapies with reduced side effects, nanotechnology offers solutions that are transforming the landscape of reproductive health care. In this expanded discussion, we will explore the role of nanotechnology in reproductive health diagnostics and treatment, including imaging techniques, drug delivery systems, targeted therapies, and advances in gene therapy and ribonucleic acid (RNA) interference (RNAi) delivery systems.[18] Imaging techniques have long been a cornerstone of diagnosing reproductive diseases, including infertility, PCOS, endometriosis, and reproductive cancers such as ovarian and endometrial cancers. Traditional imaging modalities such as magnetic resonance imaging (MRI), ultrasound, and optical imaging have provided non-invasive ways to visualize anatomical structures and detect pathological changes. However, these techniques often suffer from limitations, such as insufficient resolution or contrast, making it challenging to detect small tumors or early-stage diseases. Nanotechnology has addressed these limitations by introducing nanoparticles as contrast agents, which significantly enhance the resolution and sensitivity of imaging techniques.[19]
NANOPARTICLES AS CONTRAST AGENTS
Nanoparticles such as gold nanoparticles, iron oxide nanoparticles, and quantum dots can be used as contrast agents to improve the quality of imaging. These nanoparticles have unique optical, magnetic, or electrical properties that can enhance the contrast between healthy and diseased tissues, making it easier to localize and characterize abnormalities within the reproductive organs. For example, in MRI, iron oxide nanoparticles can enhance the magnetic properties of tissues, improving the visibility of tumors or lesions that would otherwise be difficult to detect. Similarly, gold nanoparticles are used in optical imaging techniques, such as photoacoustic imaging, to enhance the contrast of tumors, providing clinicians with more detailed and accurate information about the size, shape, and location of the pathology.[20]
IMPROVED DIAGNOSIS AND MONITORING
Nano-enhanced imaging techniques not only improve the initial diagnosis but also allow for better monitoring of disease progression and treatment efficacy. By enabling real-time visualization at the molecular level, clinicians can track how diseases such as reproductive cancers or endometriosis evolve over time. This capability is especially valuable in monitoring the response to treatments such as chemotherapy or hormone therapy, allowing for adjustments in treatment plans based on real-time data. For instance, in ovarian cancer, where early detection is crucial for successful treatment outcomes, nano-enhanced imaging can detect even minute changes in tumor size, helping clinicians make more informed decisions about the course of treatment.[21,22]
CLINICAL APPLICATIONS AND FUTURE DIRECTIONS
While nano-enhanced imaging techniques are still in the early stages of clinical application, ongoing research is focused on optimizing these technologies for widespread use. The integration of nanotechnology with artificial intelligence (AI) is also an emerging area, where AI algorithms can analyze nano-enhanced imaging data to provide more accurate diagnoses and predictive models for disease progression. As these technologies continue to develop, they hold the potential to become standard tools in the diagnosis and management of reproductive diseases.
NANOCARRIERS FOR DRUG DELIVERY IN REPRODUCTIVE DISEASES
Nanocarriers are one of the most promising applications of nanotechnology in the treatment of reproductive diseases. These nanoscale delivery systems are engineered to carry therapeutic agents directly to the site of disease, minimizing systemic side effects and improving the bioavailability of drugs.[23] This is particularly important in reproductive health, where traditional drug therapies often fail to deliver adequate concentrations of medication to the affected tissues without causing undesirable side effects. [Figure 1 and Table 2] summarize the applications of nanocarriers in the treatment of reproductive diseases.
| Study | Nanomedicine approach | Application in reproductive medicine | Key findings | References |
|---|---|---|---|---|
| Tian and Wang (2016) | Polymeric nanoparticles | Fertility-enhancing agent delivery | Controlled release of hormones for improved outcomes | Tian and Wang[24] |
| Lee and Kim (2016) | Nanoparticle platforms | Targeted therapeutics for reproductive health | Improved specificity for treatment of PCOS | Lee and Kim[25] |
| Liu et al. (2007) | Nanomedicine for imaging | Diagnosis of ovarian cancer | Enhanced imaging for early detection | Liu et al.[26] |
| Li and Wang (2014) | Nanotechnology-based drug delivery | Treatment of ovarian cancer | Nanoparticles for more effective drug delivery | Li and Wang[27] |
| Meng and Xue (2017) | Polymer-based nanoparticles | Applications in reproductive health | Targeted delivery and reduced side effects | Meng and Xue[28] |
| Kataoka and Harada (2001) | Block copolymer micelles | Hormone delivery systems | Controlled release of reproductive hormones | Kataoka et al.[29] |
| Nishiyama and Kataoka (2006) | Polymeric micelles | Gene delivery in reproductive disorders | Improved gene therapy delivery | Nishiyama and Kataoka[30] |
| Torchilin (2007) | Micellar nanocarriers | Reproductive health drug delivery | Enhanced bioavailability of infertility drugs | Torchilin[31] |
| Jain and Stylianopoulos (2010) | Nanomedicine delivery systems | Cancer treatment in reproductive organs | Improved delivery to solid tumors | Jain and Stylianopoulos[8] |
| Liu et al. (2007) | Functional nanoparticles | Diagnosis and treatment of reproductive cancers | Advanced diagnostic imaging and targeted treatment | Liu et al.[26] |
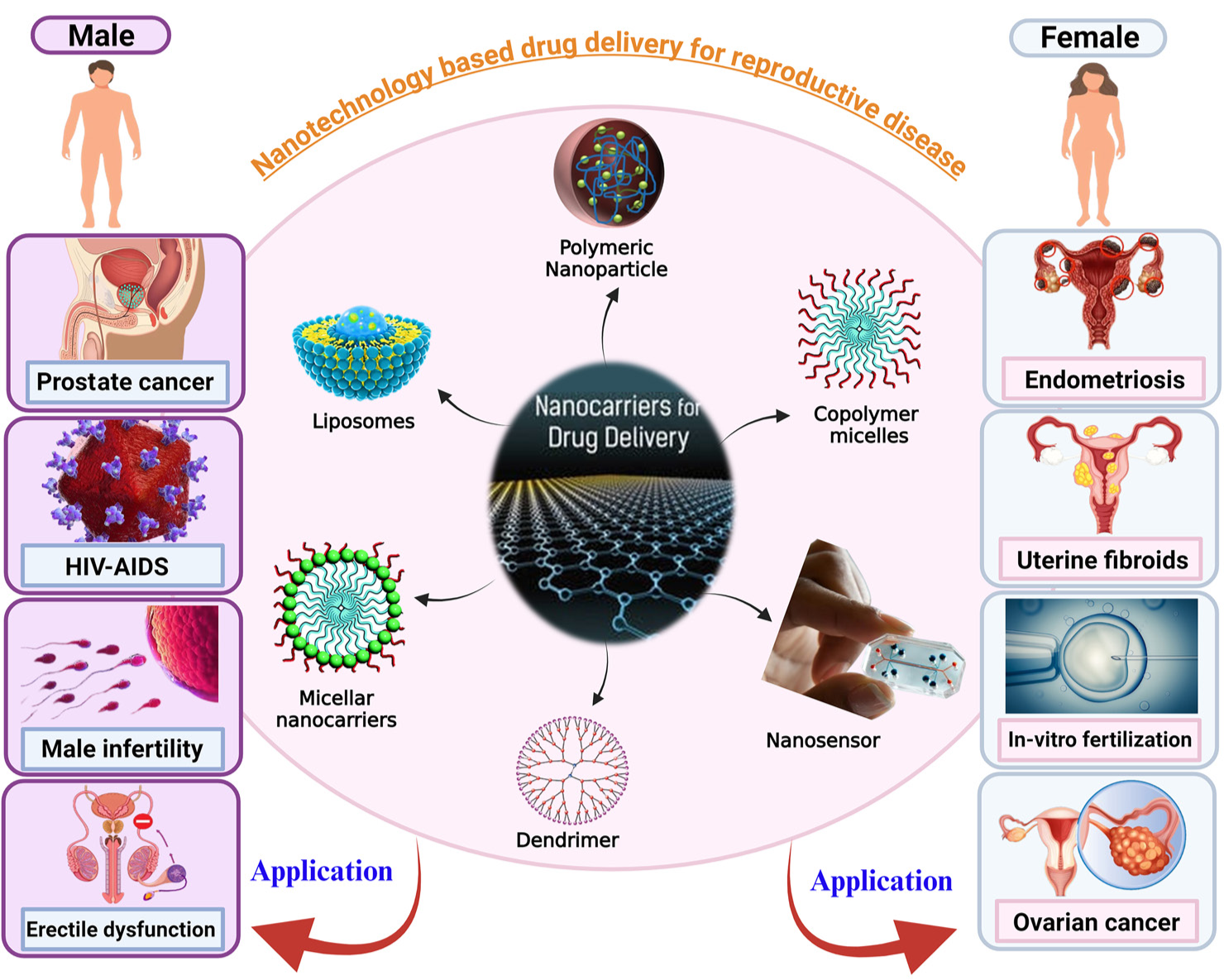
- A pictorial representation of different nanocarriers used for treatment of male and female reproductive diseases. HIV: Human immunodeficiency virus, AIDS: Aquired immunodeficiency syndrome
ADVANTAGES OF NANOCARRIERS
Nanocarriers offer several advantages over conventional drug delivery systems. First, they can be designed to release drugs in a controlled and sustained manner, ensuring that therapeutic levels of the drug are maintained for extended periods. This is particularly beneficial in treating chronic conditions like endometriosis, where long-term management of pain and inflammation is required. Second, nanocarriers can be functionalized with ligands, antibodies, or peptides that target specific receptors on diseased cells, allowing for more precise drug delivery.[32,33] This targeted approach is especially useful in treating reproductive cancers, where nanocarriers can deliver chemotherapeutic agents directly to cancer cells while sparing healthy tissues.
LIPOSOMES, MICELLES, AND DENDRIMERS
Types of nanocarriers
Several types of nanocarriers have been explored for drug delivery in reproductive health, including liposomes, micelles, dendrimers, and polymer-based nanoparticles. Each of these nanocarriers has unique properties that make them suitable for different therapeutic applications.
Liposomes are spherical vesicles composed of a lipid bilayer, which can encapsulate both hydrophilic and hydrophobic drugs. This dual capability allows liposomes to protect drugs from degradation in the body, enhancing their stability and bioavailability. In reproductive health, liposomes have been used to deliver hormones, such as progesterone and estrogen, in the treatment of infertility and hormonal imbalances. Liposomal formulations are also being developed for the delivery of chemotherapeutic agents in the treatment of ovarian and endometrial cancers.[31]
Micelles are self-assembling nanostructures that form in aqueous solutions and can encapsulate poorly water-soluble drugs. This improves the solubility and absorption of drugs that would otherwise have low bioavailability. Micelles have been explored as carriers for anti-inflammatory drugs used in the treatment of endometriosis and other inflammatory reproductive conditions.[24] By enhancing the solubility and stability of these drugs, micelles offer more efficient and targeted treatment options.
Dendrimers are highly branched, tree-like polymers that offer precise control over drug release profiles. Their unique structure allows for the attachment of multiple drug molecules or targeting ligands, making them highly versatile nanocarriers. In reproductive health, dendrimers are being investigated for their potential to deliver drugs to treat reproductive cancers, infections, and fertility disorders. Dendrimers can also be functionalized to target specific receptors on diseased cells, offering a higher level of precision in drug delivery.[34,35]
Polymer-Based nanoparticles are another class of nanocarriers that have shown promise in reproductive health. These nanoparticles are made from biocompatible and biodegradable polymers, such as poly(lactic-coglycolic acid) (PLGA), which allow for controlled and sustained drug release. Polymer-based nanoparticles are particularly useful in delivering chemotherapeutic agents to treat ovarian cancer.[28] By overcoming biological barriers, such as the blood-ovary barrier, these nanoparticles can deliver drugs more effectively to the target site, improving treatment outcomes. Table 3 summarizes the approaches of targeted drug delivery discussed above.
| Nanocarrier type | Composition | Reproductive disease | Drug delivered | Therapeutic advantage | References |
|---|---|---|---|---|---|
| Liposomes | Phospholipid bilayer | Ovarian cancer | Chemotherapeutics | Prolonged circulation, reduced toxicity | Tian and Wang,[24] Torchilin[31] |
| Micelles | Amphiphilic block copolymers | Endometriosis | Anti-inflammatory Agents | Improved solubility and absorption | Lai et al.[34] |
| Dendrimers | Branched polymers | Infertility | Hormones | Controlled drug release | Wang and Ma[35] |
| Polymer-Based Nanoparticles | PLGA, Chitosan | Reproductive Cancers | Chemotherapeutics, siRNA | Targeted delivery, sustained release | Meng and Xue,[28] Cheng et al.[36]Zhang and Zhangm,[37] Brigger et al.[38] |
PLGA: Poly(lactic-co-glycolic acid), siRNA: Small interfering RNA
TARGETED THERAPIES USING NANOPARTICLES
Targeted therapies using nanoparticles involve designing nanocarriers that specifically recognize and bind to diseased cells, reducing the risk of damaging healthy tissues. This precision in drug delivery is particularly important in reproductive health, where off-target effects can lead to complications in fertility or reproductive organ function. Nanoparticles can be functionalized with ligands, antibodies, or peptides that target specific receptors on diseased cells, offering a highly targeted approach to treatment.[36]
TARGETING OVARIAN AND ENDOMETRIAL CANCER CELLS
One of the most promising applications of targeted nanoparticle therapies is in the treatment of ovarian and endometrial cancers. These cancers are often diagnosed at advanced stages, making them difficult to treat with conventional chemotherapy. By functionalizing nanoparticles with targeting ligands that bind to receptors overexpressed on cancer cells, such as folate receptors or human epidermal growth factor receptor 2 (HER2) receptors, these therapies can deliver chemotherapeutic agents directly to the tumor site. This targeted approach reduces systemic toxicity and enhances the efficacy of the treatment.[37]
REDUCING SIDE EFFECTS AND IMPROVING OUTCOMES
Targeted nanoparticle therapies also hold the potential to reduce the side effects associated with conventional cancer treatments. For example, chemotherapy drugs often cause significant damage to healthy tissues, leading to side effects such as hair loss, nausea, and immunosuppression. By delivering drugs directly to cancer cells, targeted nanoparticle therapies minimize exposure to healthy tissues, reducing these adverse effects and improving the patient’s quality of life during treatment.[38]
GENE THERAPY AND RNAI DELIVERY SYSTEMS
Nanotechnology has also enabled advancements in gene therapy and ribonucleic acid (RNAi) delivery systems for the treatment of reproductive diseases. Gene therapy involves introducing genetic material, such as deoxyribonucleic acid (DNA) or RNA, into cells to correct genetic defects or silence disease-causing genes. RNAi is a related technique that uses small interfering RNA (siRNA) molecules to silence specific genes involved in disease processes.[26,39]
NANOPARTICLE-MEDIATED GENE DELIVERY
Nanoparticles provide an ideal platform for delivering genetic material into cells. Traditional methods of gene delivery, such as viral vectors, are often associated with safety concerns, including the risk of immune reactions or insertional mutagenesis. Nanoparticles, on the other hand, can protect genetic material from degradation in the body and facilitate its entry into target cells without triggering an immune response.[27] In reproductive health, nanoparticle-mediated gene delivery holds promise for treating genetic disorders affecting fertility, such as Turner’s syndrome or Klinefelter’s syndrome. It is also being explored as a potential therapy for correcting genetic mutations involved in reproductive cancers.
RNAI-BASED TREATMENTS
RNAi-based treatments are another exciting area of research in reproductive health.[40] Using siRNA molecules to silence disease-causing genes, RNAi therapies offer a targeted approach to treating conditions such as endometriosis or reproductive cancers. Nanoparticles can be used to deliver siRNA molecules into cells, protecting them from degradation and enhancing their uptake by target tissues. This approach is particularly promising for diseases like ovarian cancer, where overexpression of specific genes drives tumor growth.[25] By silencing these genes, RNAi therapies have the potential to halt disease progression and improve treatment outcomes.
SAFETY CONCERNS IN NANOMEDICINE FOR REPRODUCTIVE HEALTH
The rapid advancements in nanotechnology, particularly in the field of reproductive health, bring about significant safety concerns that require rigorous investigation. Nanoparticles, by virtue of their small size and distinctive physicochemical properties, interact with biological systems in ways that are not fully understood. These interactions can sometimes lead to unpredictable effects, raising concerns about the potential risks associated with nanomedicine in reproductive health.[41]
UNINTENDED INTERACTIONS WITH BIOLOGICAL SYSTEMS
Nanoparticles are typically designed to navigate the body more efficiently, crossing biological barriers and reaching target tissues more precisely than conventional drugs. However, their ability to traverse critical barriers – such as the placental barrier – poses potential risks, particularly during pregnancy.[42] The placenta acts as a shield that protects the developing fetus from harmful substances, but nanoparticles, due to their small size, may be able to bypass this natural defense. Studies have suggested that certain nanoparticles can accumulate in the placenta, potentially causing harm to the fetus by interfering with its development or triggering inflammatory responses; nanoparticles could cross the placental barrier and reach fetal tissues, raising the possibility of developmental toxicity and long-term effects on the child’s health.[43]
In addition to fetal risks, there are concerns about the accumulation of nanoparticles in reproductive tissues. Nanoparticles may not be easily cleared by the body’s natural elimination processes, leading to bioaccumulation in sensitive areas such as the ovaries, testes, or uterine lining. The long-term presence of nanoparticles in these tissues could disrupt normal cellular functions, hormonal balance, or even fertility.[44] For instance, metal-based nanoparticles such as silver or gold nanoparticles, which are commonly used for their antimicrobial or imaging properties, have been shown in some studies to induce oxidative stress and DNA damage in reproductive cells. These is need for a thorough understanding of how nanoparticles behave within reproductive organs over extended periods.[45]
TOXICITY AND IMMUNOGENICITY
The toxicological profile of nanoparticles remains a critical concern in nanomedicine. While nanoparticles are often designed to be biocompatible and non-toxic, there is still a risk that they may induce unintended immune reactions. For instance, certain nanoparticles may be recognized as foreign by the immune system, triggering inflammatory responses that could exacerbate conditions such as endometriosis or reproductive cancers.[46] Furthermore, some nanoparticles can generate reactive oxygen species, leading to oxidative stress and inflammation, which could compromise reproductive health. Nanoparticles’ interaction immune system is not always negative, however. In some cases, nanoparticles can be engineered to enhance immune responses, for example, in the context of cancer immunotherapy. Nonetheless, this immunogenic potential must be carefully controlled to prevent unintended consequences in reproductive tissues, where an overactive immune response could interfere with normal reproductive processes.
SAFE CLEARANCE AND LONG-TERM BIOCOMPATIBILITY
Another significant safety concern is the clearance of nanoparticles from the body. Nanoparticles that do not degrade or are not effectively excreted can persist in the body, leading to long-term exposure and potential toxicity. The biodistribution and clearance mechanisms of nanoparticles are influenced by several factors, including their size, shape, surface charge, and chemical composition. Biodegradable nanoparticles, such as those made from PLGA, are designed to break down into non-toxic byproducts that the body can easily eliminate. However, non-biodegradable nanoparticles, such as metal-based or carbon-based nanoparticles, may accumulate in organs and tissues over time, posing risks of chronic toxicity.[29]
The clearance of nanoparticles from e-tissues is of particular concern, as these organs are highly sensitive to chemical insults. Ensuring that nanoparticles are safely metabolized and excreted is critical to minimizing any long-term health risks. Pre-clinical studies must thoroughly evaluate the pharmacokinetics and biodistribution of nanoparticles, focusing on their potential to accumulate in reproductive organs and their impact on fertility and reproductive health over time.
NEED FOR RIGOROUS PRECLINICAL AND LONG-TERM TESTING
Given these safety concerns, rigorous pre-clinical testing and long-term studies are essential to establish the safety profile of nanomedicine in reproductive health. Animal models are commonly used in pre-clinical studies to assess the safety, biodistribution, and potential toxicity of nanoparticles. However, the results from animal studies may not always accurately predict human responses, underscoring the need for careful translation of preclinical findings into clinical practice.[30]
In addition, long-term studies that monitor nanoparticles over extended periods are crucial. This includes assessing their impact on reproductive health, fertility, and offspring development in cases where nanoparticles may cross the placental barrier. Regulatory agencies must establish stringent guidelines for the development and testing of nanomedicines in reproductive health, ensuring that any potential risks are identified and mitigated before these therapies reach the market.
ETHICAL ISSUES IN NANOTECHNOLOGY APPLICATIONS
Beyond the safety concerns, the application of nanotechnology in reproductive health raises several ethical challenges. These challenges revolve around issues of access, equity, consent, and the broader implications of manipulating human reproductive cells at the nanoscale level.
ACCESS AND EQUITY
One of the most pressing ethical issues is the potential for unequal access to advanced nanomedicine therapies. As with many cutting-edge medical technologies, there is a risk that only individuals with access to high-quality healthcare will benefit from the latest nanotechnology-based treatments. This could exacerbate existing disparities in reproductive healthcare, particularly in low- and middle-income countries, where access to even basic reproductive services is limited.[47] To ensure that the benefits of nanotechnology are desirable, policymakers and healthcare systems must prioritize making these technologies affordable and accessible to all, regardless of socioeconomic status.
ETHICAL CONCERNS IN ASSISTED REPRODUCTIVE TECHNOLOGIES (ART)
The use of nanotechnology in assisted reproductive technologies (ART) raises unique ethical concerns, particularly regarding the manipulation of human reproductive cells and embryos at the nanoscale level. Nanoparticles are being explored for use in improving the efficiency of in vitro fertilization procedures, enhancing sperm and egg quality, or delivering genetic material to embryos for gene editing purposes.[48] These applications, while promising, provoke questions about the implications of manipulating human reproduction at such a fundamental level. For example, using nanotechnology to enhance certain traits or “correct” genetic defects in embryos raises concerns about the potential for designer babies, where parents might select specific traits such as intelligence or physical appearance.
Furthermore, the use of nanotechnology in ART must be carefully regulated to ensure that ethical boundaries are respected. This includes ensuring that interventions do not cross into areas that could be considered eugenics or violate the integrity of the human reproductive process. Ethical guidelines must be developed to establish clear boundaries for the use of nanotechnology in ART, with a focus on preserving the dignity and autonomy of individuals undergoing these treatments.
INFORMED CONSENT AND PATIENT AUTONOMY
Another ethical challenge in nanomedicine is ensuring that patients provide fully informed consent when receiving nanotechnology-based treatments. Given the novelty of these therapies and the limited availability of long-term safety data, it is crucial that patients are made aware of the potential risks and benefits before consenting to treatment. In some cases, patients may feel pressured to try experimental nanomedicine therapies due to the lack of alternative treatment options, particularly in the context of reproductive health conditions such as infertility or cancer.
Healthcare providers must ensure that patients understand the experimental new treatments and that they are given the opportunity to weigh the risks and benefits carefully. Ethical guidelines should emphasize the importance of patient autonomy, ensuring that individuals are fully informed and able to make decisions that align with their values and preferences.[49]
REGULATORY FRAMEWORK FOR NANOTECHNOLOGY IN REPRODUCTIVE TREATMENTS
The regulatory landscape for nanotechnology in reproductive treatments is still in its formative stages, evolving alongside the rapid advancements in this field. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are tasked with developing comprehensive guidelines to oversee the safe and effective implementation of nanomedicine in healthcare, including its application in reproductive treatments.[50] These regulatory efforts aim to address the unique challenges that arise from the distinct properties of nanoparticles, such as their small size, high surface area, and ability to interact with biological systems in unprecedented ways.
KEY REGULATORY CONSIDERATIONS
-
Standardization of Nanoparticle Characterization
A fundamental aspect of nanomedicine regulation is the standardization of nanoparticle characterization. Nanoparticles used in reproductive treatments must undergo rigorous testing to ensure that their physical, chemical, and biological properties are well understood and consistent across different batches. This includes size distribution, surface charge, solubility, and the potential for aggregation. The characterization of these properties is critical in predicting how nanoparticles will behave in the body, especially within the sensitive reproductive system. Regulatory bodies require standardized methods for characterizing these particles to ensure reproducibility and safety during clinical use.[51]
-
Assessment of Long-Term Safety
Due to the novel interactions between nanoparticles and biological systems, long-term safety assessments are a critical regulatory concern. Nanoparticles used in reproductive health, particularly those that may cross the placental barrier or accumulate in reproductive tissues, must be studied for their long-term effects on fertility, fetal development, and overall reproductive health. Regulatory guidelines mandate extensive pre-clinical and clinical studies that include toxicology, pharmacokinetics, and biodistribution assessments over prolonged periods to ensure that nanomedicine does not pose unforeseen risks to patients.[52]
-
Clinical Trial Protocols for Nanomedicine
Establishing clear and stringent clinical trial protocols is another major focus for regulatory agencies. Nanomedicine products designed for reproductive treatments must undergo multiple phases of clinical trials to assess their safety, efficacy, and potential side effects in humans. Given the unique properties of nanoparticles, these trials must be designed to evaluate not only the therapeutic benefits but also any unintended biological interactions that may arise. Regulatory bodies such as the FDA and EMA are working to establish tailored guidelines for clinical trials involving nanotechnology, ensuring that they comprehensively address the specific risks associated with nanoparticles.[53]
-
Quality Control and Manufacturing Standards
Ensuring the quality and consistency of nanomedicine products are essential for patient safety. Regulatory agencies require manufacturers to adhere to Good Manufacturing Practices, which ensure that nanoparticle-based treatments are produced in a controlled and standardized environment. This minimizes the risk of contamination and ensures that the nanoparticles maintain their intended properties throughout the production process.[54] All these approaches are summarized in Table 4.
| Key consideration | Regulatory requirement | Description | References |
|---|---|---|---|
| Nanoparticle characterization | Standardized testing protocols | Ensures consistent size, shape, surface charge, and solubility of nanoparticles used in reproductive health applications. | Food and Drug Administration[55] |
| Long-Term safety assessment | Extensive preclinical and clinical studies | Evaluates the long-term effects of nanoparticles on reproductive tissues, fertility, and fetal development. | European Medicines Agency[56] |
| Clinical trial protocols | Tailored guidelines for nanomedicine trials | Establishes phase-specific requirements for evaluating the safety and efficacy of nanoparticles in human trials, including reproductive treatments. | European Commission[57] |
| Quality control and manufacturing | Adherence to good manufacturing practices (GMP) | Ensures quality, purity, and reproducibility of nanomedicine products through standardized manufacturing processes. | Oberdorster et al.[58] |
| Toxicology and biodistribution | Mandatory toxicology studies | Analyzes the potential toxic effects of nanoparticles and their distribution within reproductive organs and other biological systems. | Zhang et al.[59] |
CONCLUSION
Nanotechnology is ushering in a new era in reproductive healthcare, with its transformative potential spanning diagnosis, treatment, and personalized care for a wide range of reproductive diseases. This advanced technology has opened up new possibilities for enhancing early detection, improving diagnostic accuracy, and refining treatment approaches, offering hope to patients suffering from infertility, PCOS, endometriosis, and reproductive cancers. The integration of nanotechnology into reproductive health represents a significant leap forward in the precision and efficacy of medical interventions. One of the most notable contributions of nanotechnology is in diagnostics. The development of nanosensors enables the detection of biomarkers in minute concentrations within bodily fluids, providing real-time analysis that is essential for early diagnosis and effective disease management. These nanosensors can be incorporated into point-of-care devices, allowing for rapid and accurate testing, which is critical in reproductive medicine, where timely interventions can drastically improve outcomes. By detecting reproductive diseases at their earliest stages, nanosensors empower healthcare providers to make more informed decisions, facilitate early treatment, and increase the chances of successful interventions, particularly in conditions such as infertility and reproductive cancers.
Nanotechnology has also revolutionized imaging techniques. The use of nanoparticles as contrast agents in imaging modalities, such as MRI, ultrasound, and optical imaging, allows for enhanced visualization of tumors, lesions, or other pathological changes within the reproductive organs. This increased resolution at the molecular level enables clinicians to better characterize reproductive conditions, improving diagnostic accuracy and helping guide more effective treatments. In addition, the ability to track disease progression and assess treatment response with higher precision contributes to better patient outcomes.
In terms of treatment, nanocarriers have emerged as a groundbreaking advancement in drug delivery systems. Nanoparticles such as liposomes, micelles, and dendrimers are engineered to deliver drugs directly to diseased tissues, improving drug bioavailability and minimizing systemic side effects. This targeted approach is particularly valuable in treating reproductive diseases such as endometriosis, ovarian cancer, and other reproductive disorders, where precise drug delivery can significantly reduce harm to healthy tissues and enhance therapeutic efficacy.
Furthermore, polymer-based nanoparticles and other nanocarriers allow for controlled and sustained release of drugs, offering more efficient and patient-friendly treatment options.
Beyond conventional therapies, nanotechnology also paves the way for innovative approaches such as gene therapy and RNAi systems. Nanoparticles are increasingly being used to deliver genetic material, such as DNA or siRNA, to correct genetic defects or silence disease-causing genes, presenting new therapeutic options for genetic disorders affecting reproductive health. This level of precision holds immense potential for tackling previously untreatable conditions, offering hope for personalized medicine in the field of reproductive health.
However, despite the immense promise of nanotechnology, its application in reproductive medicine raises significant safety, ethical, and regulatory concerns. The potential for nanoparticles to interact with biological systems in unpredictable ways underscores the need for rigorous preclinical testing, long-term safety studies, and thorough evaluation of biocompatibility. Ensuring that nanoparticles do not cross biological barriers, such as the placenta, or accumulate in reproductive tissues is crucial to preventing unintended consequences. In addition, ethical concerns, particularly around the use of nanotechnology in ART, must be addressed with robust oversight to ensure equitable access and fully informed patient consent.
A strong regulatory framework is essential to ensure the safe integration of nanotechnology into reproductive healthcare. Regulatory agencies, such as the FDA and EMA, are working to establish comprehensive guidelines that address the unique challenges posed by nanomedicine, from standardizing nanoparticle characterization to ensuring the safety of clinical trials. Establishing these regulatory standards will be key to accelerating the safe transition of nanotechnology from the research laboratory to clinical practice, ensuring that these innovative therapies meet the highest standards of safety and efficacy.
In conclusion, nanotechnology offers unprecedented opportunities to revolutionize reproductive healthcare, providing advanced tools for early detection, precise diagnosis, and targeted treatment. While significant progress has been made, the road ahead requires continued research, stringent safety evaluations, and ethical oversight to fully harness the potential of nanotechnology in improving reproductive health. As research and clinical applications of nanotechnology continue to advance, this emerging field holds the promise of transforming reproductive medicine, offering more personalized, efficient, and effective care for patients worldwide, from bench to bedside.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- About reproductive health Georgia: Centre for Disease Control and Prevention; 2024.
- [Google Scholar]
- 1 in 6 people globally affected by infertility Geneva: World Health Organization; 2023.
- [Google Scholar]
- Ovarian stimulation: The challenges. J Hum Reproduct Sci. 2016;9:15-22.
- [CrossRef] [PubMed] [Google Scholar]
- Primary ovarian insufficiency: Update on clinical and genetic findings. Front Endocrinol (Lausanne). 2024;15:1464803.
- [CrossRef] [PubMed] [Google Scholar]
- Nanotechnology for cancer imaging: Advances, challenges, and clinical opportunities. Radiol Imaging Cancer. 2021;3:e200052.
- [CrossRef] [PubMed] [Google Scholar]
- New frontiers in nanotechnology for cancer treatment. Nat Rev Drug Discov. 2008;7:411-28.
- [CrossRef] [PubMed] [Google Scholar]
- Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653-64.
- [CrossRef] [PubMed] [Google Scholar]
- Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145-60.
- [CrossRef] [PubMed] [Google Scholar]
- Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5:709-11.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmaceutical nanotechnology for cancer therapy. Nanomedicine. 2009;4:677-86.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor site-specific silencing of NFkappaB p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer Res. 2009;69:1654-62.
- [Google Scholar]
- Nanoparticles in medicine: Therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761-9.
- [CrossRef] [PubMed] [Google Scholar]
- Nanomaterials for biosensing applications: A review. Front Chem. 2014;2:63.
- [CrossRef] [PubMed] [Google Scholar]
- Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Nat. 2012;4:34-55.
- [CrossRef] [PubMed] [Google Scholar]
- Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751-60.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310-6.
- [CrossRef] [PubMed] [Google Scholar]
- Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615-27.
- [CrossRef] [PubMed] [Google Scholar]
- Nanopreparations for mitochondria-targeted drug delivery. Int J Nanomed. 2014;9:413-5.
- [Google Scholar]
- Nanoprobes for biomedical imaging in living systems. Nano Today. 2011;6:204-20.
- [CrossRef] [Google Scholar]
- Advances in cancer therapy using targeted nanoparticles. Nat Rev Cancer. 2013;13:622-33.
- [Google Scholar]
- Nanoparticle platforms for targeted therapeutics in reproductive health. Mol Pharm. 2016;13:2173-84.
- [CrossRef] [PubMed] [Google Scholar]
- Nanomedicine for drug delivery and imaging: A promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. Int J Cancer. 2007;120:2527-37.
- [CrossRef] [PubMed] [Google Scholar]
- Nanotechnology-based drug delivery systems for the treatment of ovarian cancer. J Control Release. 2014;193:273-82.
- [Google Scholar]
- Nanomedicine and polymer-based nanoparticles in reproductive health. J Biomed Nanotechnol. 2017;13:111-21.
- [Google Scholar]
- Block copolymer micelles for drug delivery: Design, characterization, and biological significance. Adv Drug Deliv Rev. 2001;47:113-31.
- [CrossRef] [PubMed] [Google Scholar]
- Current state and perspectives of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Ther. 2006;112:630-48.
- [CrossRef] [PubMed] [Google Scholar]
- Micellar nanocarriers: Pharmaceutical perspectives. Pharm Res. 2007;24:1-16.
- [CrossRef] [PubMed] [Google Scholar]
- Functionalized gold nanoparticles and their biomedical applications. Nanomaterials. 2012;3:108-49.
- [Google Scholar]
- Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36-48.
- [CrossRef] [PubMed] [Google Scholar]
- Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158-71.
- [CrossRef] [PubMed] [Google Scholar]
- Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903-10.
- [CrossRef] [PubMed] [Google Scholar]
- Nanoparticles in cancer therapy: Opportunities and challenges. Adv Mater. 2012;24:1026-41.
- [Google Scholar]
- Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631-51.
- [CrossRef] [PubMed] [Google Scholar]
- Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771-82.
- [CrossRef] [PubMed] [Google Scholar]
- Nanocarriers in the treatment of cancer: A patent review. Expert Opin Ther Patents. 2014;24:1101-12.
- [Google Scholar]
- Transport of molecules in the tumor interstitium: A review. Cancer Res. 1987;47:3039-51.
- [Google Scholar]
- Targeted drug delivery using polymeric nanocarriers. Adv Drug Deliv Rev. 2004;56:1037-52.
- [Google Scholar]
- Nanoparticle-based drug delivery systems for the treatment of cancer. J Nanosci Nanotechnol. 2011;11:93-105.
- [Google Scholar]
- Targeted drug delivery to tumors: Myths, reality, and possibility. J Control Release. 2011;153:198-205.
- [CrossRef] [PubMed] [Google Scholar]
- Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm Res. 2010;27:2569-89.
- [CrossRef] [PubMed] [Google Scholar]
- Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol. 2010;624:25-37.
- [CrossRef] [PubMed] [Google Scholar]
- Review paper: Chitosan derivatives as promising materials for controlled drug delivery. J Biomater Appl. 2011;25:291-311.
- [Google Scholar]
- Polymeric micelles for delivery of poorly water-soluble compounds. Crit Rev Ther Drug Carrier Syst. 2003;20:357-403.
- [CrossRef] [PubMed] [Google Scholar]
- Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469-78.
- [CrossRef] [PubMed] [Google Scholar]
- Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33:2373-87.
- [CrossRef] [PubMed] [Google Scholar]
- The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomedicine. 2013;9:1-14.
- [CrossRef] [PubMed] [Google Scholar]
- Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329-47.
- [CrossRef] [PubMed] [Google Scholar]
- Nanomedicine--challenge and perspectives. Angew Chem Int Ed Engl. 2009;48:872-97.
- [CrossRef] [PubMed] [Google Scholar]
- Scientific opinion on the risk assessment of nanomaterials Belgium: European Commission; 2020.
- [Google Scholar]
- Toxicology of nanoparticles: A historical perspective. Nanotoxicology. 2018;12:1-23.
- [Google Scholar]
- Nanoparticles in medicine: Therapeutic applications and developments. Nat Rev Drug Discov. 2021;20:201-20.
- [Google Scholar]






