Translate this page into:
Exploring the role of Withania somnifera in male reproductive health: Insights from laboratory and clinical study

*Corresponding author: Raghav Kumar Mishra, Department of Zoology, Male Reproductive Physiology Lab, Institute of Science, Banaras Hindu University, Varanasi, Uttar Pradesh, India. raghav.mishra1@bhu.ac.in
-
Received: ,
Accepted: ,
How to cite this article: Yadav A, Mishra RK. Exploring the role of Withania somnifera in male reproductive health: Insights from laboratory and clinical study. J Reprod Healthc Med. 2025;6:3. doi: 10.25259/JRHM_27_2024
Abstract
Various therapy options are evolving globally to address male reproductive dysfunction resulting from hormone imbalances, altered neuroendocrine interactions, infections, lifestyle changes, etc. Modern therapies, including hormonal therapy and assisted reproductive technologies, are expensive and have meager success rates (up to 30%) with side effects; yet, herbal medicines are recognized as an alternative therapeutic approach for male reproductive dysfunctions. The positive benefits of oral consumption of the roots of the perennial herb Withania somnifera (WS) (Ashwagandha) on the semen quantity and quality of reproductively challenged men have been previously investigated. The oral consumption of Ashwagandha roots has been shown to enhance sperm motility and numbers, impede lipid peroxidation, elevate antioxidant enzymes, and modulate the reproductive axis (hypothalamic–pituitary–testicular axes). The exact molecular cross-talk behind these actions remains elusive. This review examined the role of natural medicines on male reproductive health, offering a comprehensive analysis of various clinical and laboratory studies pertaining to WS. It showed a direct oxido-inflammatory-apoptotic mechanism that alleviates oxidative damages, apoptosis, and inflammation, alongside an indirect mechanism involving a dopaminergic-gamma-aminobutyric acid (GABAergic)-cholinergic secretion-associated as well as through hypothalamic–pituitary–gonadal-hypothalamic–pituitary–adrenal axes pathway that enhances hormonal equilibrium through interactions among endocrine glands to enhance male sexual function and fertility. In addition, it addressed how WS ameliorates various risk factor-associated reproductive dysfunctions and enhances overall male reproductive health.
Keywords
Erectile dysfunction
Male infertility
Male reproductive health
Testis
Withania somnifera
INTRODUCTION
Male reproductive dysfunction is defined as the inability of a male to father a child after 12 months of unprotected, regular intercourse or issues with sexual satisfaction due to inadequate arousal, erection, or premature ejaculation. Globally, around 48.5 million couples face infertility, with male factors contributing to over 50% of cases.[1] Erectile dysfunction alone is expected to affect 322 million men by 2025.[2] Male reproductive dysfunctions are multifactorial, with causes such as hormonal imbalances, neuroendocrine disruptions, infections, physical, lifestyle, varicocele, psycho-social, and environmental factors, while around 50% remain idiopathic.[3]
Hormonal imbalances associated with male infertility and impotence, such as low testosterone or disrupted levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), can impair sperm production and libido.[4] Physical issues such as varicocele (enlarged veins in the scrotum), blockages, or infections in the reproductive tract also interfere with fertility. Environmental factors, including exposure to pesticides, heavy metals, and endocrine-disrupting chemicals, further impact reproductive health.[5] Psychological stress is increasingly recognized as a significant consequence of modern lifestyle choices, impacting both mental and physical health. The interplay between lifestyle habits and stress responses is complex, with various factors, such as smoking, excessive alcohol consumption, poor diet, and chronic stress, contributing to oxidative stress, which damages sperm DNA and lowers sperm quality.[6]
Psychological stress significantly impacts male reproductive health, affecting both hormonal regulation and sperm quality. Stress activates the hypothalamic–pituitary–adrenal (HPA) axis, leading to elevated cortisol levels, which can interfere with gonadotropin secretion, particularly LH and FSH.[7] These hormones are crucial for testosterone production and spermatogenesis, and disruptions in their release can impair testosterone synthesis and decrease sperm production.[8] Chronic stress alters the pulsatile release of gonadotropin-releasing hormone (GnRH), further reducing LH and FSH secretion. Behavioral changes due to stress also affect reproductive health. Stress often reduces sexual desire, contributes to erectile dysfunction, and impairs ejaculatory function, which can further exacerbate psychological distress.[9]
In addition, stress alters the oxidative balance by increasing reactive oxygen species (ROS) production. Elevated ROS can damage sperm DNA, proteins, and lipids, causing poor sperm motility, abnormal morphology, and, in severe cases, DNA fragmentation.[10,11] Oxidative stress is increasingly recognized as a mechanism behind stress-induced infertility, prompting interest in antioxidant interventions to mitigate its effects on sperm quality.[12,13] Addressing both psychological well-being and oxidative stress is essential for improving and maintaining optimal male reproductive health.
Current treatments for reproductive disorders include stress management, hormone therapy, costly medications, and assisted reproductive technologies (ART), with success rates of around 30%. Although, these interventions often have substantial costs and side effects. Consequently, herbal therapies are being explored for male infertility treatment due to their affordability and minimal side effects.[14]
Treatment options
Treatment options for male infertility and erectile dysfunction include a range of medical and surgical interventions tailored to specific causes. For infertility, hormonal therapies, such as gonadotropins and testosterone, address hormonal imbalances impacting sperm production. Advanced solutions such as in vitro fertilization (IVF) and intracytoplasmic sperm injection help in severe cases. Erectile dysfunction treatments include oral medications, like phosphodiesterase type 5A (PDE5A) inhibitors (e.g., sildenafil), hormonal therapy, vacuum erection devices, and penile injections.[15] For persistent cases, surgical options like penile implants provide effective results. However, these treatments have side effects: Hormonal therapies may cause mood swings, acne, and cardiovascular issues, while PDE5A inhibitors can lead to headaches and, rarely, vision or hearing loss.[16,17] Surgical procedures risk infection, bleeding, and scar tissue, and ART procedures like IVF are emotionally and financially demanding, with potential risks of multiple pregnancies.[18]
The potential side effects and high demands of conventional treatments for male infertility and erectile dysfunction highlight the need for alternative medicines, like Ayurvedic treatments, as complementary or standalone options. Traditional medicines offer natural, holistic options with fewer adverse effects. For centuries, herbal formulations have been central to medical treatments across Asia, South America, and Africa, capturing scientific interest for their potential in treating various conditions.[19] The World Health Organization estimates that 80% of the global population in developing countries relies on herbal remedies.[20] Ayurveda, often termed the “science of life,” is an ancient Indian medical system practiced alongside modern therapies, claiming to promote comprehensive health and longevity.[21] Its herbal remedies have shown efficacy in managing conditions such as diabetes, gastritis, hypertension, and reproductive disorders.[19] Integrating Ayurveda with conventional reproductive health care may provide safer, more accessible solutions, potentially reducing dependence on intensive therapies and enhancing patient quality of life.[22]
This review provides a comprehensive evaluation of the role of natural medicines in male reproductive health, with a particular emphasis on Withania somnifera (WS), based on an extensive analysis of both clinical and experimental studies.
COMMON AYURVEDIC HERBS USED FOR MALE REPRODUCTIVE ISSUES
Ayurveda, the traditional Indian system of medicine, employs a variety of herbs renowned for their rejuvenating and aphrodisiac properties to address male reproductive issues, particularly infertility. These herbs enhance sperm quality, balance hormones, and improve overall sexual health and fertility through their multifaceted bioactive compounds activating diverse mechanisms.
Shatavari (Asparagus racemosus) improves spermatogenesis, stress management, and hormonal balance,[23] while Kapikacchu (Mucuna pruriens) improves sperm count, motility, and quality by enhancing testosterone and essential neurotransmitters.[24,25] Safed Musli (Chlorophytum borivilianum) is used for its aphrodisiac properties, boosting sperm count and stamina,[26] and Gokshura (Tribulus terrestris) elevates testosterone, facilitating spermatogenesis and reproductive vitality.[27] Shilajit, rich in fulvic acid, enhances sperm quality and protects against oxidative damage.[28] Amla (Emblica officinalis), a potent antioxidant, safeguards sperm DNA and improves semen quality[29], while Tongkat Ali (Eurycoma longifolia) boosts testosterone and addresses sexual dysfunction.[30,31] Licorice (Glycyrrhiza glabra) supports hormonal balance, spermatogenesis, and overall reproductive health, making these herbs integral to addressing male infertility and promoting sexual wellness.[32] Ashwagandha (WS) is a well-known adaptogenic herb in Ayurveda. It mitigates stress, which is crucial for maintaining reproductive health, by reducing cortisol levels, which can impair testosterone synthesis and spermatogenesis. Ashwagandha has been demonstrated to improve testosterone levels, sperm count, and motility, thereby enhancing libido and overall vitality. Its antioxidant properties, primarily due to withanolides, suppress lipid peroxidation (LPO) in sperm cells, addressing idiopathic male infertility and restoring hormonal balance in stressed individuals.[12,33,34]
ASHWAGANDHA (WS)
WS (L.) Dunal, an evergreen perennial herb belonging to the family Solanaceae, is known by several names with specific connotations, including “Indian winter cherry,” “Asgandh,” “Indian ginseng,” “Punir,” and “Asgand.”[35] The “somnifera” species name in Latin translates to “sleep-inducer,” attributed to its remarkable anti-stress properties. The popular name for it is “Ashwagandha,” which comes from the Sanskrit words “ashwa” (horse) and “gandha” (smell) since the roots have an aroma similar to that of a wet horse. Because of the similarities between its traditional applications and the pharmacological effects of Korean ginseng tea, it is also known as Indian ginseng.[36]
Ashwagandha, in Ayurveda, referred to as the “Sattvic Kapha Rasayana,” has been extensively used for its anti-stress, narcotic, astringent, tonic, anti-carbuncle, diuretic properties and in the treatment of sleeplessness, worms, leucoderma, goiter, piles, mental breakdown, reproductive issues, and constipation.[34] In 78 A.D., Withania, referred to as “Asgand,” is also cited in Kitab-al-Hashaish in the Unani medical system authored by Dioscorides. WS is listed as an official natural medicine in the Indian Pharmacopoeia of 1985.[37] The plant’s root has been widely used in Ayurvedic and Unani medicinal systems. Roots are shown to have amino acids, volatile oils, alkaloids (0.13–0.31%), glycosides, starch, and steroids.[38] Furthermore, the leaves and root are effective in managing inflammation and fever; the flowers function as astringents and diuretics; the fruits are utilized for skin ulcers, tumors, and carbuncles; and the seeds and roots significantly contribute to increasing sperm count, supporting testicular physiology, and enhancing sexual health.[39] In addition, in Ayurveda WS has been extensively used for the treatment of endocrinological and male reproductive health problems.[12]
WS: DISTRIBUTION AND TRADITIONAL USES
The most extensively dispersed species in its genus, WS, is often found in arid areas that span the Middle East, Arabian Peninsula, Indian subcontinent, and tropical Africa to South Africa. WS is mostly cultivated as a medicinal crop in the Indian subcontinent because of its fleshy roots, which are abundant in a variety of phytoconstituents with important pharmacological qualities.[40,41] The plant is extensively found in the arid regions of India, particularly in Punjab, West Bengal, Maharashtra, Uttar Pradesh, Gujarat, and Rajasthan.[42] The various ethnomedical uses of WS are summarized in Table 1, supporting male reproductive health.
| Place | Local names | Plant part | Traditional use | References |
|---|---|---|---|---|
| Andhra Pradesh | Penneru; Pannerugadda | Root | Sperm quantity; Aphrodisiac | Pingali et al.[43]Divya et al.[44] |
| Chhattisgarh | Ashwagandha | Root; Leaf | Gonorrhoea; Male reproductive health | Hsu et al.[45]Jain and Singh[46] |
| Karnataka | Ashwagandha | Root; Leaf | Aphrodisiac | Ghatapanadi et al.[47] |
| Madhya Pradesh | Ashwagandha | Root | Male sexual health | Jain et al.[48], Patil et al.[49] |
| Maharashtra | Ashwagandha; Askand; Dhorgunj; Aasoodkand | Root; Leaf | Weakness and nocturnal emission Sperm quantity and count, Aphrodisiacs, impotency |
Dushing and Patil[50]Shaikh et al.[51]Bhogaonkar and Kadam[52] |
| Orissa | Ashwagandha | Root, Leaf | Infertility Sperm count |
Mallick et al.[53]Singh[54] |
| Tamil Nadu | Amukkara | Root; Leaf | Sexual Vigor Aphrodisiac |
Tariq and Ifham[55] |
| Telangana | Ashwagandha; Pecnneru gadda; Domma dolu gadda | Root | Fertility; Impotency | Sreeramulu et al.[56]Sureshbabu and Ramakrishna[57] |
| Uttar Pradesh | Ashwagandha; Asgandh | Root; Leaf; Seeds | Aphrodisiacs Sexual organs weakness, Antioxidant activity Sexual diseases |
Singh and Singh,[58]Khan and Khan,[59]Kumar et al.[60] |
| West Bengal | Ashwagandha | Root | Impotency | Dey et al.[61] |
| Pakistan | Asgandh; Kotilal; Aksn | Roots; Leaf; Whole plant; Seeds; Green berries; Flowers | Aphrodisiac, ant- inflammations Sexual illness; infertility and tonic. |
Muhammad and Khan,[62]Nisar et al.[63]Qureshi et al.[64]Sher et al.[65]Mahmood et al.[66]Shah et al.[67] |
| Bangladesh | Ashwagandha | Roots; Leaf; Whole plant | Penile bleeding Libido and sexual arousal |
Shah et al.[68]Islam et al.[69] |
CHEMOTYPES OF WS
The chemotypes of WS are classified according to variations in withanolide steroidal lactones, which are influenced by genetic factors and geographic distribution. Three primary chemotypes (I, II, and III) have been identified [Figure 1].[70]
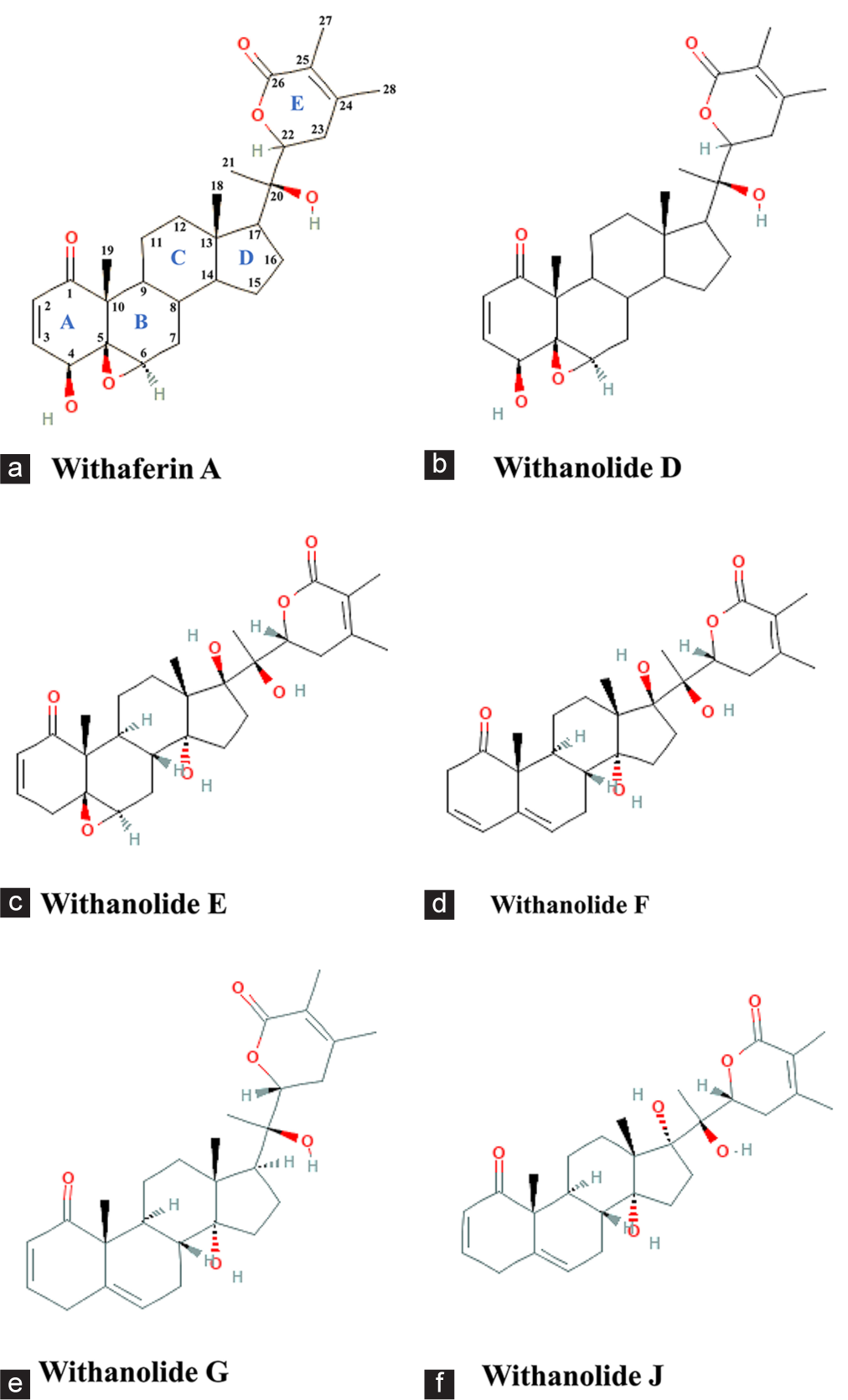
- Chemical structures representing the different classes of chemotypes found in Withania somnifera. (a) Withaferin A representing the chemotype I, (b) Withanolide D representing the chemotype II; (c-f) Withanolide E, F, G, and J representing the chemotype III. O-Oxygen, H-Hydrogen. (Source: PubChem)
Chemotype I
It is characterized by its ability to introduce hydroxyl groups at various carbon positions. The defining features include substituents in the A/B rings, such as a 4b-hydroxyl group and a 5,6-b-epoxy system, coupled with the absence of a hydroxyl group at the C-20 position of the side chain. Withaferin A is a representative compound of this chemotype.
Chemotype II
Chemotype II is distinguished by the presence of withanolide D as the major withanolide. It shares structural features with Chemotype I, including the 4b-hydroxyl group and the 5,6-b-epoxy system. However, it differs in the presence of a hydroxyl group at the C-20 position.
Chemotype III
This chemotype contains compounds with a hydroxyl group at the C-20 position and is further divided into two groups based on the stereochemistry of the side chain. The first group includes compounds with an a-oriented side chain, like withanolides E and F, while the second group comprises compounds with a b-oriented side chain, like withanolides G and J. In addition, a hydroxyl group at the C-17 position is common to both withanolides E and J. Notably, Chemotype III also features three distinct double bonds located in the A, B, and C rings of certain compounds.
In India, WS predominantly consists of Chemotype I; however, it contains both withaferin A and withanone as the major withanolides. Furthermore, Indian samples have been found to exhibit a hybrid chemotype that incorporates characteristics of both Chemotypes I and II. This hybrid chemotype is characterized by the presence of withaferin A and withanolide D as the primary withanolides.[71]
WS: PHYTOCONSTITUENTS AND PHARMACOLOGICAL PROPERTIES
Phytochemical analyses of WS have revealed a diverse array of bioactive constituents across its various parts, including over 12 alkaloids, approximately 40 withanolides, and multiple sitoindosides. Among the chemicals that are pharmacologically active in WS, the most important contributors are withanolides, which belong to the class of steroidal compounds known as ergostane-type compounds. The distinguishing feature of these withanolides is the presence of a q-lactone ring that is situated between the C-26 and C-22 atoms, as well as an oxidized C-1 position. It is important to note that withanolides are exclusive to the Solanaceae family, particularly within the Withania genus. Both withanolide D and withaferin A have been recognized as the most important withanolides discovered in this plant. In addition to exhibiting anti-inflammatory, antitumor, and immunosuppressive properties, withanolides act as potent antioxidants, contributing to neuroprotective effects and enhancing fertility.[34,72,73] The distribution of phytochemicals specific to each part of WS is detailed in Table 2. A different class of molecules from WS is outlined in Figure 2.
| Plant segments | Phytoconstituents | References |
|---|---|---|
| Fruits | Tocopherols, fatty acids, elaidic acid, linoleic acid, palmitic acid, tetracosanoic acid, oleic acid, hydrocarbons (squalene), sterols, and Withanamides A-I. | Saleem et al.[73]Bhatia et al.[74] |
| Leaves | Ashwagandhine, Anaferine (bis (2-piperidylmethyl) ketone), 27-acetoxy-3-oxo-witha- 1,4,24-trienolide, mesoanaferine, anahygrine, choline, Pubesenolide, Jaborosalactone D, 17-hydroxy withaferin A, isopelletierine, 3α-Tigloyloxtropine, pseudotropine, cuscohygrine, 3-Tropyltigloate, hygrine, dl-isopelletierine, somniferine, withanine; withananine, withasomnine, visamine, pseudowithanine, Withanolide D, N, O, P; Withanolides G–M, Withanolide F, T, and U; Withanoside IV, physagulin, and withanoside VI. 5b, 6b-epoxy-4b- hydroxy-1-oxo-witha-2,16,24- Trienolide; (22R)- 5b- formyl-6b, 27-dihydroxyl-1-oxo-4- norwith-24-enolide; 2,3-dihydrowithaferin A, 5a, 17a-dihydroxy-6a, 7a-epoxy-1-oxo-3b Osulfatewitha-24-enolide. 6a-Chloro-5b, 17a-dihydroxywithaferin A, 6a, 7a-epoxy-3b, 5a, 17a-trihydroxy-1-oxo-witha-24- Enolide, 6a-chloro-5b-hydroxywithaferin A 3-methoxy-2,3-dihydrowithaferin A withanoside X, viscosalactone B, 27- hydroxywithanolide B. 4b, 27-dihydroxy-L-oxo- 22R-witha-2,5,24-trienolide 2,3-didehydrosomnifericin, hentriacontane, tropine, 27-deoxywithaferin A. |
Paul et al.[34]Saleem et al.[73]Misra et al.[75] |
| Leaves and Roots | withanone, 27-hydroxy withanone, 17-hydroxy withaferin A, Physagulin, 17-hydroxy-27-deoxy withaferin A, withanolide D, 27-hydroxy withanolide B, 27-deoxywithaferin A, Withaferin-A, Withastramonolide, Withanolide-A. | Saleem et al.[73] Dhar et al.[76] |
| Roots | Anaferine, Ashwagandhanolide, β-sitosterol and d-glycoside, choline, Withanolide A, Pseudotropine, 16b-Acetoxy-6, 7a-epoxy-5a– hydroxy-1-oxowitha2, 17 (20), 24-trienolide isopelletierine, 3α-tigloyloxtropine tropine, dl-isopelletierine-3-tropyltigloate, cuscohygrine, hygrine, anahygrine, somniferine, mesoanaferine, withanine, Withanosides I, II, III, IV, V, VI, and VII. visamine, withananine, hentriacontane, withasomnine, along with pyrazole derivatives pseudowithanine and ashwagandhine, Withasomniferol A, B, and C, Physagulin D and coagulin Q. 5,7a-Epoxy-6a, 20a-dihydroxy-1-oxowitha-2,24-Dienolide. |
Saleem et al.[73]Misra et al.[75] |
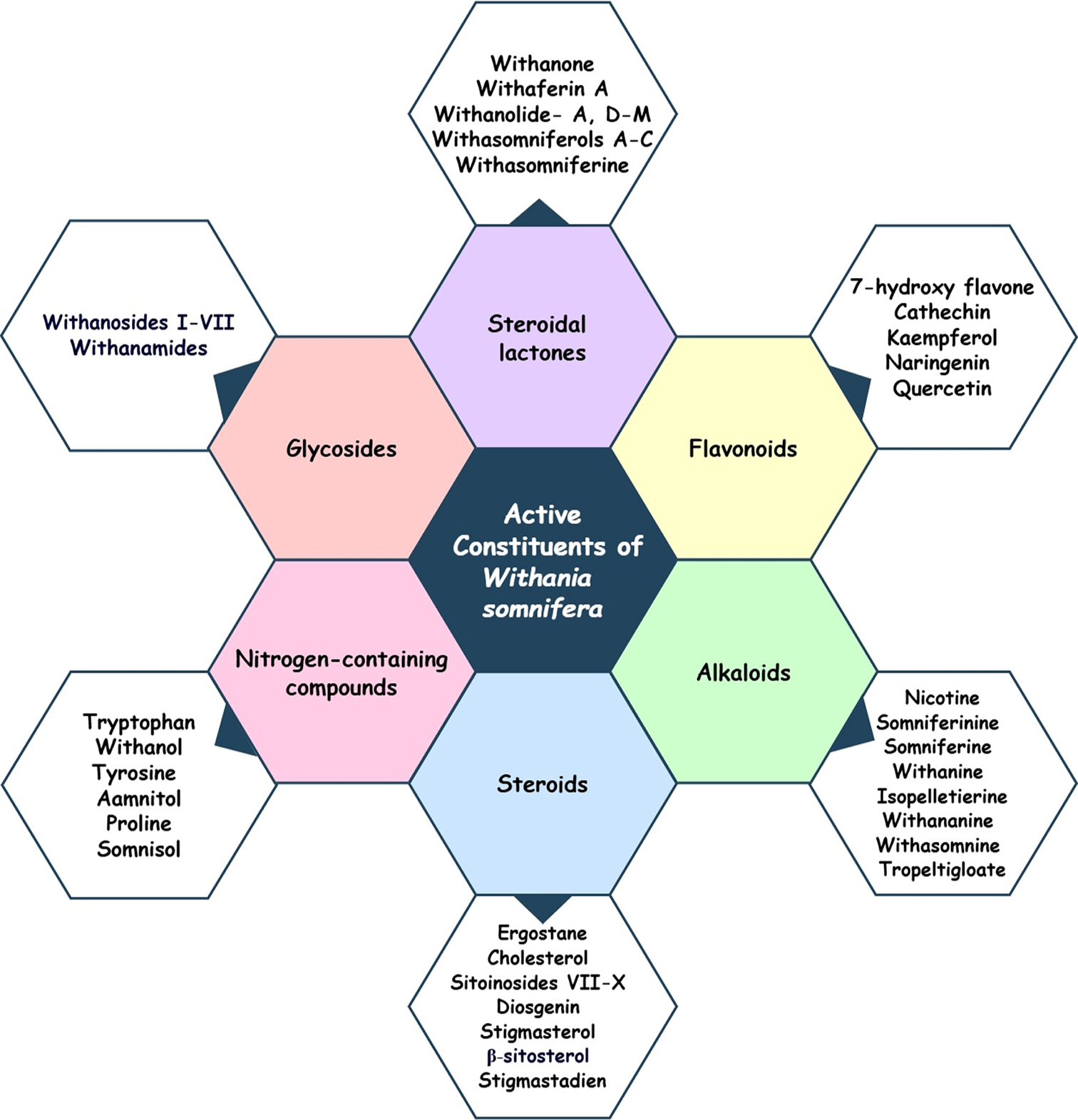
- Illustration showing different phytoconstituents of Withania somnifera. Figures is created by using Draw .io.
The therapeutic potential of WS has been validated by numerous preclinical trials and pharmacological investigations. Multiple in vitro and in vivo studies have shown the bioactivities of different components of WS. Figure 3 provides an overview of the pharmacological activities of WS, both as an independent treatment and in conjunction with other formulations.
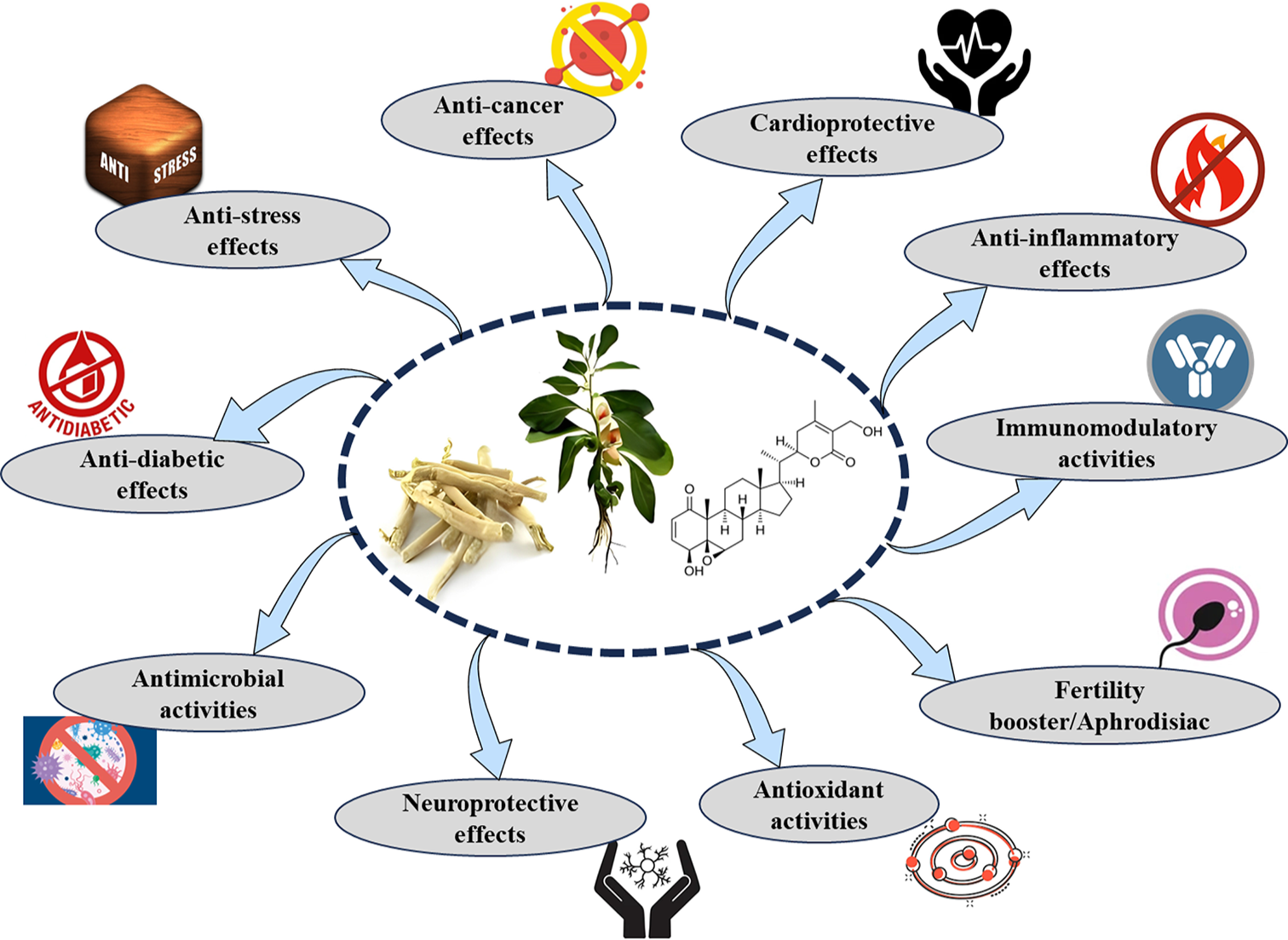
- Illustration showing various pharmacological properties of Withania somnifera. Figure created by BioRender.
WS AND MALE REPRODUCTIVE HEALTH
WS has been shown to enhance sexual activities and satisfaction, as evidenced by studies conducted in both humans and animals. The impact of WS therapy on copulatory performance, libido, and sexual satisfaction has been recorded in these studies. It additionally enhances the quality and quantity of sperm and reproductive hormones in both healthy individuals and those with infertility issues. The steroidal lactones, the principal pharmacologically active constituents of WS, account for the majority of its effects.[12]
WS LEAVES, FRUITS AND STEM
The reproductive effects of WS have been extensively studied using various extracts from its leaves, fruits, and stems. These studies explore the impact of WS on hormonal levels, testicular function, sperm parameters, and antioxidant activity across different animal models, revealing both therapeutic benefits and potential adverse effects.
A lyophilized aqueous extract of WS leaves (47 mg/100 g body weight [BW]) administered to male Wistar rats for 6 days through a 10% aqueous solution significantly improved reproductive parameters. The treatment elevated LH levels while reducing FSH and testosterone (T) levels. Notable increases were observed in testicular weight, seminiferous tubule diameter, and seminiferous tubular cell layer counts. In addition, spermatogenesis was markedly enhanced, indicating improved testicular function and reproductive health.[77]
The 2% ethanolic extract of fresh leaves of WS, injected subcutaneously into male albino mice for 15 days, resulted in a significant reduction in total and mitochondrial LPO and a marked increase in sperm count. In addition, the treatment led to the recovery of degenerative changes in both the testis and epididymis, as observed in histological examinations.[78]
Glycowithanolides extracted from leaves of WS, injected subcutaneously into Swiss albino male mice at a dose of 20 mg/kg BW for 20 days, resulted in increased epididymal sperm count, as well as the weights of the testes, epididymis, and body. Normal testicular histology was restored in D-galactose-exposed mice.[79] A similar study by Walvekar et al. [80] showed a marked reduction in total and mitochondrial LPO and fluorescence product levels in the testes, epididymis, and seminal vesicles.
Furthermore, a 70% methanolic extract of leaves and roots of WS, administered orally to male albino rats at a dose of 100 mg/kg BW for 15–30 days, effectively countered acephate-induced (75 mg/kg BW/day for 15 and 30) damage by restoring antioxidant enzyme levels (catalase, superoxide dismutase [SOD], and glutathione), reducing malondialdehyde levels, and improving hormonal concentrations (testosterone, FSH, and LH). Histological examination showed improved testicular architecture, with seminiferous tubules and intertubular spaces nearing normal and a marked recovery in sperm count and motility.[81]
A 50% ethanolic extract of WS fruit, administered orally to male albino rats at a dose of 50 mg/kg BW per day for 60 days, significantly reduced sperm motility and density in both testicular and cauda epididymal sperm. The treatment also resulted in notable reductions in the weights of the testes, seminal vesicles, and other accessory reproductive organs. Histological analysis revealed degenerative changes in the seminiferous tubules, including germinal epithelium degeneration, a marked decline in spermatogenic elements, and a substantial increase in intertubular space.[82]
Similarly, the hydroalcoholic extract of WS fruit, administered orally at a dose of 200 mg/kg BW for 60 days, caused significant reductions in the number of primary and secondary spermatocytes, mature sperm, and the weights of the testes and accessory reproductive organs. The treatment also increased the incidence of abnormal seminiferous tubules and led to significant reductions in protein, sialic acid, fructose, and ascorbic acid levels (P < 0.01, P < 0.001) compared to controls.[83]
Furthermore, oral administration of ethanolic stem extract of WS at doses of 25 mg and 50 mg/kg/day for 20 and 60 days resulted in significant decreases in sperm density, sperm motility, and fertility rate, as well as reductions in the weights of the testes, epididymis, and seminal vesicles. Histological examinations showed decreased spermatogenesis, reduced seminiferous tubule size, and diminished Leydig cell nuclei diameter. However, no significant changes were observed in testosterone, FSH levels, sperm morphology, serum biochemistry, hematological parameters, or BW compared to the control group.[84]
WS ROOT
The processes by which WS influences the male reproductive system can be categorized into two main pathways: the oxido-inflammatory-apoptotic axis and the neuro-endocrine axis. The oxido–inflammatory–apoptotic axis involves the regulation of antioxidant enzymes and their essential cofactors for optimal function alongside a balance between pro- and anti-inflammatory/apoptotic factors. The neuroendocrine axis primarily includes the effects of WS on the dopaminergic, GABAergic, and cholinergic systems, as well as the hypothalamic–pituitary–gonadal (HPG) axis, and its anti-stress properties through the HPA axis. WS extract is metabolized into its principal constituents-withanone, withanolide D, withaferin A, and other withanolide derivatives. These phytochemicals either exert direct effects on male reproductive organs or indirectly influence neuromodulators and neuro-endocrine homeostasis to enhance male reproductive physiology. The proposed general pathway of action of WS is outlined in Figure 4.
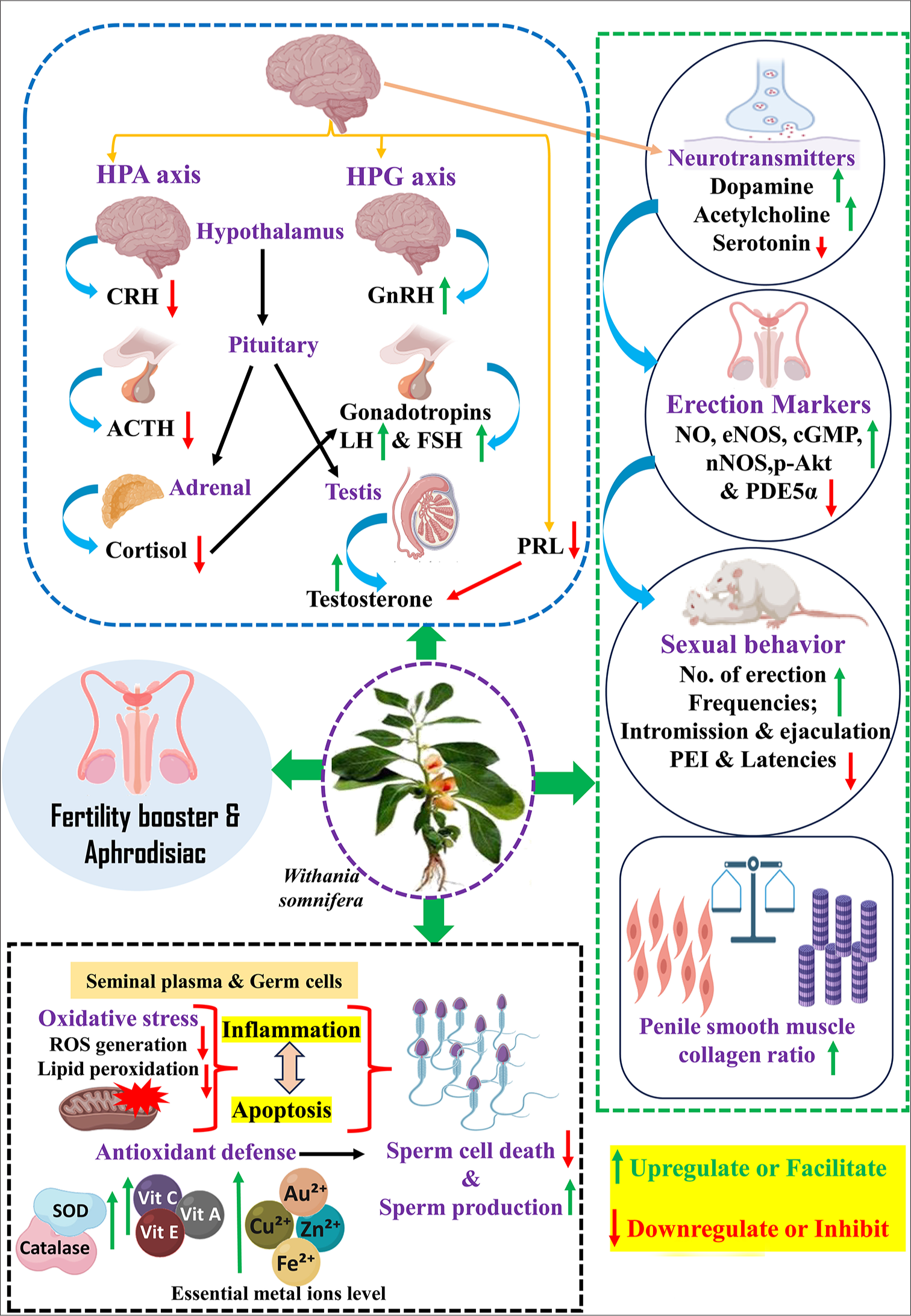
- Illustration showing the possible mechanism of Withania somnifera on male reproductive health. HPG: Hypothalamic pituitary gonad, HPA: Hypothalamic pituitary adrenal, CRH: Corticotropin-releasing hormones, ACTH: Adrenocorticotropic hormone, PRL: Prolactin, LH: Luteinizing hormones, FSH: Follicle-stimulating hormones, ROS: Reactive oxygen species, SOD: Superoxide dismutase, GnRH: Gonadotropin-releasing hormone, NO: Nitric oxide, NOS: Nitric oxide synthase, PEI: Post-ejaculatory intervals, PDE-5a: Phosphodiesterase 5 alpha. (Source: Created with BioRender)
WS ROOT AS A FERTILITY BOOSTER
Semen from infertile men exhibits reduced antioxidant activity. This decreased total antioxidant capacity results from either diminished activity or concentration of functional antioxidant enzymes or elevated levels of ROS that exceed the natural antioxidant capacity. Supplementation with WS root powder (5 g/day for 3 months) in infertile men with normozoospermia led to decreased seminal ROS production and improved sperm concentration.[85] Multiple studies assessing the impact of Withania on semen quality in infertile men evaluated antioxidant enzyme activity by quantifying protein carbonyl groups and LPO in semen, indirectly reflecting the enzymatic activities of SOD and catalase. Compared to pretreatment, LPO levels decreased following Withania supplementation [Table 3].[85-87] Compounds in Withania extract can donate electrons and interrupt the chain reaction of damaging free radicals, thereby reducing the overall ROS burden.[88]
Studies also indicated that Withania modulates semen metal ions such as zinc, copper, selenium, and iron,[89] which are essential cofactors for SOD, glutathione peroxidase, and catalase, modulating oxidative status and apoptosis in spermatozoa,[85] as illustrated in Figure 4. A dose of Withania root at 300 mg/kg BW demonstrates anti-apoptotic and anti-inflammatory effects, significantly reducing apoptotic cells and testicular tumor necrosis factor-alpha and nuclear factor kappa B (NF-kB) expression in cadmium-exposed male rats.[90] Sahin et al. [91] reported that an aqueous root extract of Withania at a dose of 300 mg/kg BW increases the expression of antioxidant enzyme synthesis-associated factors, nuclear factor erythroid 2-related factor and heme oxygenase-1 and decreases testicular NF-kB expression in a rat model, suggesting its antioxidative and anti-inflammatory roles. Another recent study showed that Withania at a dose of 500 mg/kg significantly increases testicular B-cell lymphoma 2 (BCL-2) expression and decreases Bax expression in cyclophosphamide-exposed rats, indicating its anti-apoptotic effects.[92] Overall, reports suggest that WS operates through an oxido-inflammatory-apoptotic mechanism that alleviates oxidative damage, apoptosis, and inflammation-related male fertility issues.
Semen comprises spermatozoa suspended in secretions known as seminal fluid or seminal plasma.[93] Seminal plasma contains several chemical constituents, including albumin, inorganic ions, hormones, peptides, and enzymes, which indirectly or directly influence sperm viability.[94] Based on observed differences in metabolites between infertile and fertile men, Gupta et al. [95] assessed the effectiveness of Withania on seminal metabolites in infertile men using high-resolution proton nuclear magnetic resonance spectroscopy. In their study, infertile men received a daily dose of 5 g of Withania root extract for 3 months. Indices associated with sperm quality and amino acid derivatives were evaluated. Outcomes, including sperm motility, concentration, LPO levels, and hormone concentrations, were measured to assess fertility status before and during therapy. These amino acid derivatives also served as indicators of enzymatic activity for lactate dehydrogenase (LDH), alanine transaminase, isocitrate dehydrogenase, and aspartate transaminase, as they are the products of these enzyme processes. Gupta et al.[95] found that post-treatment metabolite levels aligned with those found in fertile men, suggesting that Withania may be an effective initial intervention for managing fertility issues. An increase in lactate content post-treatment may be attributed to the significant levels of LDH and lactate in the Withania root.[96] Lactate, a byproduct of LDH-regulated processes, correlates with improved sperm viability and motility.[94] WS enhances levels of lactate, histidine, alanine, phenylalanine, and citrate in seminal fluid, potentially impacting sperm metabolism and spermatogenesis.
In addition, Withania (200 mg/kg) supplementation reduced sperm morphological abnormalities in alcohol-exposed rats.[97] Afrodet Plus, a herbal formulation containing Withania and other natural ingredients, administered at 90 mg/kg for 21 days, improved semen quality in rats.[98]
| Source | Activity | Model | Mode of action | References |
|---|---|---|---|---|
| Root extract | Spermatogenic activity | Human | Increase in semen volume, sperm count, and motility | Ambiye et al.[99] |
| Root powder | Spermatogenic activity | Human | An increase in sperm concentration in normozoospermia enhances sperm concentration and motility under stress. | Mahdi et al.[87] |
| Ethanolic root extract | Ameliorative effect in arsenic-associated infertility | Charles Foster rats | Improved sperm count, morphology, and progressive motility. | Kumar et al.[89] |
| Ethanolic root extract | Ameliorative effect on infertility associated with alcohol intake | Wistar rats | Increases sperm count, improves motility and reduces morphological abnormalities. | Bhargavan et al.[97] |
| Root extract (methanolic) | Spermatogenic activity | Sprague–Dawley rats | Enhances semen quality, contributing to improved sperm production, motility, and morphology. | Sahin et al.[91] |
| In Afrodet plus tablet | Spermatogenic activity | Holtzman rats | Improved sperm count | Dhumal et al.[98] |
| Root extract | Spermatogenic activity in improving fertility | Human | Increases semen volume and improved sperm concentration, motility, and count in asthenozoospermia, oligozoospermia, and normozoospermia. | Ahmad et al.[86] |
| Root powder | Anti-oxidant | Human | Decreases ROS generation in normozoospermia, oligozoospermia, and asthenozoospermia | Shukla et al.[85] |
| Root powder | Anti-oxidant | Human | Increase in the level of antioxidant vitamins in asthenozoospermia, oligozoospermia, and normozoospermia. | Ahmad et al.[86] |
| Root powder | Anti-oxidant | Human | Decrease in lipid peroxidation in seminal plasma under psychological stress and infertile cases. | Mahdi et al.[87] |
| Root powder | Antioxidant | Human | Increase in the level of essential metals in seminal plasma in normozoospermia, oligozoospermia, and asthenozoospermia. | Shukla et al.[85] |
| Root powder | Anti-oxidant | Human | Increase the activity SOD, Catalase in the seminal plasma | Mahdi et al.[87] |
| Root powder | Hormonal balance | Human | Increase in serum testosterone (T), LH, and FSH levels in normozoospermia, asthenozoospermia, and oligozoospermia | Ahmad et al.[86]Mahdi et al.[87] |
| Root powder | Hormonal balance | Human | Decrease in serum prolactin level under stress and in normozoospermia, asthenozoospermia, and oligozoospermia | Ahmad et al.[86]Mahdi et al.[87] |
| Root powder | Enhanced metabolic activity in the seminal plasma | Human | Repair of disturbed seminal metabolites lactate, citrate, histidine, alanine, phenylalanine, and glycerylphosphorylcholine (GPC) and improve semen quality | Gupta et al.[95] |
| Root powder | Enhanced metabolic activity in the seminal plasma | Human | Increase in lactate dehydrogenase (LDH) and isocitrate dehydrogenase (IDH) levels in oligozoospermia and asthenozoospermia | Gupta et al.[95] |
| Root extract | Sexual health | Human (Randomized, double-blind, placebo-controlled study) | Significant improvement in the total Derogatis interview for sexual functioning in men (DISF-M) scores | Chauhan et al.[100] |
| Root extract | Sexual health | Human (A randomized controlled trial) | Significant improvement in sexual well-being, accompanied by an increase in serum testosterone levels. | Chauhan et al.[100] |
| Root powder | Sexual arousal and potency | Sexually sluggish male rats | WS ameliorates the Nitric oxide/Cyclic guanosine monophosphate/Phosphodiesterase 5 alpha (PDE5α) pathway of penile erection. | Yadav and Mishra[101] |
ROS: Reactive oxygen species, SOD: Superoxide dismutase, LH: Luteinizing hormone, FSH: Follicle-stimulating hormone, LDH: Lactate dehydrogenase, PDE5α: Phosphodiesterase type 5A
WS ROOT AS NEURO-ENDOCRINE MODULATOR
In addition to oxidative stress, inflammation and apoptosis, additional factors, including altered neuromodulators/neurotransmitters (dopamine, serotonin, acetylcholine, and prolactin [PRL]) and hormonal imbalances due to physiological, pathological, or psychological reasons, are associated with male reproductive dysfunction. Stress-related secretion, especially glucocorticoids, adversely affects the HPG axis and, therefore, spermatogenesis.[102] Hypothalamic GnRH activates the pituitary to secrete LH and FSH. Both subsequently influence the testis, influencing testosterone biosynthesis and spermatogenesis. Consequently, spermatogenesis is adversely impacted when the HPG axis is disturbed by hormones including GnIH, cortisol/corticosterone, and PRL.[8] The root extract of Withania, recognized as an adaptogen, facilitates homeostasis by mitigating the stress response and normalizing cortisol levels, partially relieving fertility issues. Previous findings proved the adaptogenic properties of WS by assessing its impact on infertile males who experienced psychological and environmental risk factors (smokers) or had unexplained infertility. The Withania root powder (5 g/day) was supplemented for 90 days, causing a reduction in cortisol levels.[87] The therapy group had normalized cortisol levels, which correlated with elevated testosterone and LH levels and reduced PRL and FSH levels.[87]
These hormone levels were similar to those of healthy men. The aforementioned research assessed pregnancy results in people administered Withania, demonstrating a mean enhancement of 14% relative to the control group. A prior analogous study examined the sex hormone levels of infertile men following a 3-month treatment with 5 g of WS root powder daily, combined with milk, revealing an elevation in LH and testosterone levels, alongside a reduction in PRL and FSH.[86] The data indicate that Withania may modulate serum sex hormone levels by modulating the HPG axis, hence improving fertility. The modulation of the HPG and HPA axes may occur at the levels of the pituitary, testis, adrenal, or hypothalamus. Numerous research indicates that the root powder of Withania exerts its effects at the hypothalamus level.[103-105] Kataria et al.[105] found that aqueous leaf extract of Withania enhanced GnRH neuronal activity through its differentiation and increased GnRH secretion in hypothalamic GnV-3 cells. The researchers suggested that the augmentation of GnRH neuronal activity resulted from the GABA-mimetic properties of Withania. The same group validated this concept through later in vitro research using hypothalamus slices from mouse brains. Withania methanolic extract affects GnRH neuronal activity by directly stimulating membrane GABA receptors instead of action potential-linked pathways.[104] In the central nervous system, GABA is primarily recognized as an inhibitory role; however, most mature GnRH neurons display an atypical characteristic, being activated by GABA stimulation.[106,107] Consequently, it may be inferred that WS influences the control of GnRH neurons in the hypothalamus, promoting its release and subsequently stimulating the synthesis of hormones FSH, LH, and testosterone. Moreover, despite the use of entire leaf or root extracts in this research, it is widely posited that the principal bioactive chemicals are Withaferin A, Withanolide, and Withanone.[103,108] If root extract of Withania influences the hypothalamus, findings from other research would corroborate those that indicated the normalization of pituitary and testicular hormone secretions and fertility status [outlined in Figure 4].[8] In vivo, investigations must be undertaken to comprehensively elucidate the intricate pathways through which Withania enhances the fertility of infertile males. Ambiye et al. [99] found that the root extract of Withania increased sperm motility and semen volume as well as LH and testosterone levels.
Sahin et al. [91] reported that an aqueous root extract of WS at a dose of 300 mg/kg BW significantly increased erection frequency, mounting, and licking behavior in rats. Furthermore, WS combined with clarified cow butter was found to be safe at doses up to 2000 mg/kg BW. At doses of 150 and 300 mg/kg BW, the treatment exhibited a dose-dependent increase (P < 0.01 and P < 0.05, respectively) in mounting and intromission frequency, anogenital sniffing, and genital grooming, highlighting its potential aphrodisiac properties. In vitro studies demonstrated substantial relaxation of the corpus cavernosum smooth muscle across all doses in a dose-dependent manner. In addition, molecular modeling investigations supported these findings by indicating an interaction with phosphodiesterase-5A as a potential molecular target.[109]
A rodent model of a psychologically stressed, sexually sluggish male showed that purified root powder of WS modulated the neuro-endocrine secretions and facilitated the penile erection markers and mating indices, which reflected in terms of improved neuromodulators secretion (dopamine, acetylcholine) hormones (LH, FSH, and testosterone), penile nitric oxide synthase (NOS), nitric oxide (NO), cyclic guanosine monophosphate (cGMP), phospho Ak strain transforming (p-Akt) expression, penile smooth muscle collagen ratio, and frequencies of mount intromission and ejaculation while suppresses the inhibitory markers such as serum serotonin and corticosterone, penile PDE5A expression, and latencies of mount, intromission and post-ejaculatory intervals, suggesting WS root powder improve the sexual arousal and erection [Table 3].[101]
CONCLUSION
WS, or Ashwagandha is a perennial herb utilized for centuries within traditional medical systems, especially in Ayurvedic practices. Throughout the years, studies have been carried out to explore the diverse impacts of Ashwagandha, revealing that it offers numerous advantages for various organ systems. It is crucial to note that investigations associated with Ashwagandha are still in progress, and additional investigations are necessary to validate its possible therapeutic applications and to establish the ideal dosages and durations. Furthermore, it is crucial to evaluate the safety of Withania, especially when it is taken alongside other supplements or in formulations. Consequently, investigation, especially through clinical trials, is essential to gain a deeper understanding of the possible advantages and drawbacks of utilizing Withania as a therapeutic agent. Current findings indicate that Withania root is a botanical substance with diverse effects. However, it is essential to consistently update the understanding of these raw forms, particularly concerning their potential applications in disease treatment and, most importantly, their safe utilization. The results indicate that Withania could possess therapeutic benefits, particularly concerning reproductive disorders, by targeting the neuromodulators-HPG-HPA axes and countering the oxidative stress, inflammation, and apoptosis to improve sperm indices, sexual arousal, and erection efficiency. However, few studies demonstrate the spermicidal effects of stems and fruits. While there is evidence indicating the possible therapeutic applications of Withania, the underlying mechanisms through which it operates remain very little studied. Hence, in the future, determining the precise mechanisms of action for Withania will be crucial for developing a more effective and targeted therapeutic approach.
Acknowledgments
RKM acknowledges the Indian Council of Medical Research (ICMR) for its support, and AY acknowledges IoE-Banaras Hindu University for RJP-PDF.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI. However, biorender application was utilized for image creation.
Financial support and sponsorship: Indian Council of Medical Research (ICMR) and Institute of Eminence, Banaras Hindu University (IoE-BHU).
References
- National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and associated factors of moderate to severe erectile dysfunction among adult men in Malaysia. Sci Rep. 2023;13:21483.
- [CrossRef] [PubMed] [Google Scholar]
- Congruence of quality of life among infertile men and women: Findings from a couple-based study. Hum Reprod. 2009;24:2151-7.
- [CrossRef] [PubMed] [Google Scholar]
- Male reproductive hormones and semen quality. Asian Pac J Reprod. 2019;8:189.
- [CrossRef] [Google Scholar]
- Impact of endocrine disrupting chemicals (EDCs) on reproductive health of human. Proc Zool Soc. 2022;75:16-30.
- [CrossRef] [Google Scholar]
- Lifestyle causes of male infertility. Arab J Urol. 2018;16:10-20.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J Neuroendocrinol. 2018;30:e12590.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of psychological stress on male fertility. Nat Rev Urol. 2015;12:373-82.
- [CrossRef] [PubMed] [Google Scholar]
- Sub-chronic restraint stress suppresses sexual potency and erection efficiency by targeting the hypothalamic-pituitary-testicular axis and the nitric oxide/cyclic guanosine monophosphate/phosphodiesterase 5a pathway in adult rats. Neuroendocrinology. 2023;113:442-56.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of oxidative stress in male infertility. Front Mol Biosci. 2022;8:799294.
- [CrossRef] [PubMed] [Google Scholar]
- Relation between seminal quality and oxidative balance in sperm cells. Acta Urol Port. 2016;33:6-15.
- [CrossRef] [Google Scholar]
- Role of Withania somnifera (Ashwagandha) in the management of male infertility. Reprod Biomed Online. 2018;36:311-26.
- [CrossRef] [PubMed] [Google Scholar]
- Sub-chronic restraint stress exposure in adult rats: An insight into possible inhibitory mechanism on testicular function in relation to germ cell dynamics. Andrologia. 2022;54:e14575.
- [CrossRef] [Google Scholar]
- Management of male erectile dysfunction: From the past to the future. Front Endocrinol (Lausanne). 2023;14:1148834.
- [CrossRef] [PubMed] [Google Scholar]
- PDE5 inhibitors-Pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol. 2018;175:2554-65.
- [CrossRef] [PubMed] [Google Scholar]
- Frontiers in hormone therapy for male infertility. Transl Androl Urol. 2018;7:S353-66.
- [CrossRef] [PubMed] [Google Scholar]
- What are the risks of the assisted reproductive technologies (ART) and how can they be minimized? Reprod Med Biol. 2013;12:151-8.
- [CrossRef] [PubMed] [Google Scholar]
- A compilation of bioactive compounds from Ayurveda. Bioinformation. 2008;3:100-10.
- [CrossRef] [PubMed] [Google Scholar]
- Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1-93.
- [CrossRef] [PubMed] [Google Scholar]
- Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid Based Complement Alternat Med. 2013;2013:376327.
- [CrossRef] [PubMed] [Google Scholar]
- Integrating ayurveda with modern medicine for enhanced patient care: Analysis of realities. Physician. 2024;9:1-6.
- [CrossRef] [Google Scholar]
- Effect of Asparagus racemosus on sexual dysfunction in hyperglycemic male rats. Pharm Biol. 2009;47:390-95.
- [CrossRef] [Google Scholar]
- Mucuna pruriens improves male fertility by its action on the hypothalamus-pituitary-gonadal axis. Fertil Steril. 2009;92:1934-40.
- [CrossRef] [PubMed] [Google Scholar]
- Mucuna pruriens reduces stress and improves the quality of semen in infertile men. Evid Based Complement Alternat Med. 2010;7:137-44.
- [CrossRef] [PubMed] [Google Scholar]
- Standardised extract of safed musli (Chlorophytum borivilianum) increases aphrodisiac potential besides being safe in male Wistar rats. Andrologia. 2016;48:1236-43.
- [CrossRef] [PubMed] [Google Scholar]
- The profertility and aphrodisiac activities of Tribulus terrestris L.: Evidence from meta-analyses. Andrologia. 2023;2023:7118431.
- [CrossRef] [Google Scholar]
- Shilajit mitigates chemotherapeutic drug-induced testicular toxicity: Study on testicular germ cell dynamics, steroidogenesis modulation, and Nrf-2/Keap-1 signaling. J Ayurveda Integr Med. 2024;15:100930.
- [CrossRef] [PubMed] [Google Scholar]
- Emblica officinalis Garten fruits extract ameliorates reproductive injury and oxidative testicular toxicity induced by chlorpyrifos in male rats. Springerplus. 2013;2:541.
- [CrossRef] [PubMed] [Google Scholar]
- Eurycoma longifolia as a potential adoptogen of male sexual health: A systematic review on clinical studies. Chin J Nat Med. 2017;15:71-80.
- [CrossRef] [PubMed] [Google Scholar]
- Review on a traditional herbal medicine, Eurycoma longifolia Jack (Tongkat Ali): Its traditional uses, chemistry, evidence-based pharmacology and toxicology. Molecules. 2016;21:331.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of licorice on sex hormones and the reproductive system. Nutrition. 2022;103-104:111727.
- [CrossRef] [PubMed] [Google Scholar]
- Indian folklore medicine in managing men's health and wellness. Andrologia. 2016;48:894-907.
- [CrossRef] [PubMed] [Google Scholar]
- Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed Pharmacother. 2021;143:112175.
- [CrossRef] [PubMed] [Google Scholar]
- Withania somnifera Ethnobotany, pharmacology, and therapeutic functions In: Bagchi D, ed. Sustained energy for enhanced human functions and activity (1st ed). Massachusetts: Academic Press; 2017. p. :137-54.
- [CrossRef] [Google Scholar]
- Pharmacologic overview of Withania somnifera the Indian Ginseng. Cell Mol Life Sci. 2015;72:4445-60.
- [CrossRef] [PubMed] [Google Scholar]
- Phytochemical and pharmacological profile of Withania somnifera Dunal: A review. J Appl Pharm Sci. 2012;2:170-75.
- [Google Scholar]
- Withania somnifera dunal. Part II: Alkaloidal constituents and their chemical characterization. Ind J Pharm. 1955;17:158-61.
- [Google Scholar]
- An overview on ashwagandha: A Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med. 2011;8:208-13.
- [CrossRef] [PubMed] [Google Scholar]
- Mabberley's plant-book: A portable dictionary of plants, their classification and uses. (4th ed). Cambridge, EN: Cambridge University Press; 2017. p. :973-82.
- [CrossRef] [Google Scholar]
- Steroidal lactones from Withania somnifera an ancient plant for novel medicine. Molecules. 2009;14:2373-93.
- [CrossRef] [PubMed] [Google Scholar]
- Withania somnifera An Indian ginseng. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1093-105.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of standardized aqueous extract of Withania somnifera on tests of cognitive and psychomotor performance in healthy human participants. Pharmacognosy Res. 2014;6:12-8.
- [CrossRef] [PubMed] [Google Scholar]
- Ethno-medicinal plants used in east Godavari district, Andhra Pradesh, India. Int J Pharm Sci Res. 2015;5:293-300.
- [Google Scholar]
- Identification of withaferin a as a potential candidate for anti-cancer therapy in non-small cell lung cancer. Cancers (Basel). 2019;11:1003.
- [CrossRef] [PubMed] [Google Scholar]
- Traditional medicinal practices among the tribal people of Raigarh (Chhatisgarh), India. Indian J Nat Prod Resour. 2024;11:109-15.
- [Google Scholar]
- Documentation of folk knowledge on medicinal plants of Gulbarga district, Karnataka. Indian J Tradit Knowl. 2011;102:349-53.
- [Google Scholar]
- Traditional phytotherapy of Balaghat district, Madhya Pradesh, India. Indian J Tradit Knowl. 2011;102:334-38.
- [Google Scholar]
- Traditional medicolore in Badwani district (MP) India. J Phytol. 2010;2:49-53.
- [CrossRef] [Google Scholar]
- Studies on ethnomedicine in Buldhana district of Maharashtra (India) J Phytol. 2010;2:35-41.
- [Google Scholar]
- Ethnobotanical study of folk medicinal plants used by villagers in Nanded district of Maharashtra (India) Int J Ayurvedic Herb Med. 2014;4:1585-95.
- [Google Scholar]
- Ethnopharmacology of Banjara tribe of Umarkhed taluka, district Yavatmal, Maharashtra for reproductive disorders. Indian J Tradit Knowl. 2006;5:336-41.
- [Google Scholar]
- Study of ethnomedicinal values of some shrubs in Rourkela steel city and its surroundings, Sundargarh, Odisha. Int J Appl Biol Pharm Technol. 2014;5:123-30.
- [Google Scholar]
- Less known ethnomedicinal uses of some plants from Sundargarh, Mayurbhanj, Angul and Balangir districts of Odisha, India. Nelumbo. 2012;54:172-81.
- [CrossRef] [Google Scholar]
- Ethnobotanical survey of medicinal plants in Yelagiri Hills of Tamil Nadu. Res J Pharm Technol. 2013;6:652-54.
- [Google Scholar]
- Ethno-botanico-medicine for common human ailments in Nalgonda and Warangal districts of Telangana, Andhra Pradesh, India. Ann Plant Sci. 2013;2:220-29.
- [Google Scholar]
- Traditional botanical knowledge of local people of Anantagiri and Dhamagundam forest area, Vikarabad district Telangana state. J Sci Innov Res. 2018;7:92-9.
- [CrossRef] [Google Scholar]
- An ethnobotanical study of medicinal plants in Chandauli District of Uttar Pradesh, India. J Ethnopharmacol. 2009;121:324-9.
- [CrossRef] [PubMed] [Google Scholar]
- Herbal folklores for male sexual disorders and debilities in western Uttar Pradesh. Indian J Tradit Knowl. 2005;43:317-24.
- [Google Scholar]
- Ethnomedicinal plants uses to cure different human diseases by rural and tribal peoples of Hathras district of Uttar Pradesh. J Pharmacogn Phytochem. 2017;6:346-48.
- [Google Scholar]
- Documentation of ethno-medicinal practices: A case study on tribal forest fringe dwellers of Terai West Bengal in India. J Appl Nat Sci. 2015;7:822-27.
- [CrossRef] [Google Scholar]
- An ethnomedicinal inventory of plants used for family planning and sex diseases in Samahni valley, Pakistan. Indian J Tradit Knowl. 2008;72:277-83.
- [Google Scholar]
- Ethno-medicinal uses of plants from district Bahawalpur, Pakistan. Curr Res J Biol Sci. 2014;6:183-90.
- [CrossRef] [Google Scholar]
- Medicoethnobotanical inventory of tehsil Chakwal, Pakistan. Pak J Bot. 2009;41:529-38.
- [Google Scholar]
- Traditional use of medicinal plants among Kalasha, Ismaeli and Sunni groups in Chitral District, Khyber Pakhtunkhwa province, Pakistan. J Ethnopharmacol. 2016;188:57-69.
- [CrossRef] [PubMed] [Google Scholar]
- Indigenous knowledge of medicinal plants from Gujranwala district, Pakistan. J Ethnopharmacol. 2013;148:714-23.
- [CrossRef] [PubMed] [Google Scholar]
- Ethnobotanical study of medicinal plants of semi-tribal Area of Makerwal & Gulla Khel (Lying between Khyber Pakhtunkhwa and Punjab Provinces), Pakistan. Am J Plant Sci. 2013;4:98-116.
- [CrossRef] [Google Scholar]
- Phytotherapeutic practices of a folk medicinal practitioner in Dinajpur district, Bangladesh. J Appl Pharm Sci. 2017;7:161-5.
- [Google Scholar]
- An ethnobotanical study of medicinal plants used by tribal and native people of Madhupur forest area, Bangladesh. J Ethnopharmacol. 2014;151:921-30.
- [CrossRef] [PubMed] [Google Scholar]
- Withania somnifera (L.) Dunal-Modern perspectives of an ancient Rasayana from Ayurveda. J Ethnopharmacol. 2021;264:113157.
- [CrossRef] [PubMed] [Google Scholar]
- Withania somnifera (Linn.) Dunal: A review of chemical and pharmacological diversity. Phytochem Rev. 2017;16:953-87.
- [CrossRef] [Google Scholar]
- In vitro propagation of Withania somnifera and isolation of withanolides with immunosuppressive activity. Planta Med. 2001;67:146-9.
- [CrossRef] [PubMed] [Google Scholar]
- Withania somnifera L.: Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran J Basic Med Sci. 2020;23:1501-26.
- [Google Scholar]
- Metabolic profiling for studying chemotype variations in Withania somnifera (L.) Dunal fruits using GC-MS and NMR spectroscopy. Phytochemistry. 2013;93:105-15.
- [CrossRef] [PubMed] [Google Scholar]
- Withanolides from Withania somnifera roots. Phytochemistry. 2008;69:1000-4.
- [CrossRef] [PubMed] [Google Scholar]
- Phytochemical and genetic analysis in selected chemotypes of Withania somnifera. Phytochemistry. 2006;67:2269-76.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of aqueous extracts of Cynomorium coccineum and Withania somnifera on testicular development in immature Wistar rats. J Ethnopharmacol. 2001;75:1-4.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effect of spermatogenic activity of Withania somnifera (Ashwagandha) in galactose stressed mice. Ann Biol Res. 2012;3:4159-65.
- [Google Scholar]
- Protective effects of glycowithanolides on antioxidative enzymes in testes and accessory reproductive organs of D-galactose induced stressed mice. Int J Curr Microbiol Appl Sci. 2014;3:458-64.
- [Google Scholar]
- Effects of glycowithanolides on lipid peroxidation and lipofuscinogenesis in male reproductive organs of mice. Iran J Reprod Med. 2013;11:711-6.
- [Google Scholar]
- Ameliorating effect of Withania somnifera on acephate administered male albino rats. Afr J Pharmacy Pharmacol. 2013;7:1554-9.
- [CrossRef] [Google Scholar]
- Evaluation of antifertility activity of Withania somnifera in male albino rats. Fertil Steril. 2008;90:S18.
- [CrossRef] [Google Scholar]
- Control of fertility in male Wistar rats treated with hydroalcholic extract of Withinia somnifera fruits. Int J Pharm Biol Sci. 2013;7:1-14.
- [Google Scholar]
- Spermicidal activity and antifertility activity of ethanolic extract of Withania somnifera in male albino rats. Int J Pharm Sci Rev Res. 2013;21:227-32.
- [Google Scholar]
- Withania somnifera improves semen quality by combating oxidative stress and cell death and improving essential metal concentrations. Reprod Biomed Online. 2011;22:421-7.
- [CrossRef] [PubMed] [Google Scholar]
- Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile males. Fertil Steril. 2010;94:989-96.
- [CrossRef] [PubMed] [Google Scholar]
- Withania somnifera improves semen quality in stress-related male fertility. Evid Based Complement Alternat Med. 2011;2011:576962.
- [CrossRef] [PubMed] [Google Scholar]
- Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): A review. Altern Med Rev. 2000;5:334-46.
- [Google Scholar]
- Phytoremedial effect of Withania somnifera against arsenic-induced testicular toxicity in Charles Foster rats. Avicenna J Phytomed. 2015;5:355-64.
- [Google Scholar]
- Protective effect of Withania somnifera (Linn.) on cadmium-induced oxidative injury in rat testis. Phytopharmacology. 2013;4:269-90.
- [Google Scholar]
- Comparative evaluation of the sexual functions and NF-kB and Nrf2 pathways of some aphrodisiac herbal extracts in male rats. BMC Complement Altern Med. 2016;16:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effects of Withania somnifera against cyclophosphamide-induced testicular damage in rats. Clin Exp Reprod Med. 2024;51:205-12.
- [CrossRef] [PubMed] [Google Scholar]
- Seminal plasma: An essential attribute to spermatozoa. J Androl. 2012;33:536-51.
- [CrossRef] [PubMed] [Google Scholar]
- Enzymatic and electrolytic profiles of human semen. Prostate. 1988;12:263-9.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of Withania somnifera on seminal plasma metabolites of infertile males: A proton NMR study at 800 MHz. J Ethnopharmacol. 2013;149:208-14.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochemistry. 2010;71:1085-94.
- [CrossRef] [PubMed] [Google Scholar]
- The protective effect of Withania somnifera against oxidative damage caused by ethanol in the testes of adult male rats. Int J Basic Amp Clin Pharmacol. 2015;4:1104-09.
- [CrossRef] [Google Scholar]
- Efficacy and safety of a herbo-mineral ayurvedic formulation “Afrodet Plus(®)” in male rats. J Ayurveda Integr Med. 2013;4:158-64.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical evaluation of the spermatogenic activity of the root extract of ashwagandha (Withania somnifera) in oligospermic males: A Pilot Study. Evid Based Complement Alternat Med. 2013;2013:571420.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of standardized root extract of ashwagandha (Withania somnifera) on well-being and sexual performance in adult males: A randomized controlled trial. Health Sci Rep. 2022;5:e741.
- [CrossRef] [PubMed] [Google Scholar]
- Withania somnifera ameliorates sexual arousal and impotence in stressed sexually sluggish male rats by modulating neurotransmitters and NO/cGMP/PDE5a pathway. J Ethnopharmacol. 2024;318:116971.
- [CrossRef] [PubMed] [Google Scholar]
- Excessive dietary calcium in the disruption of structural and functional status of adult male reproductive system in rat with possible mechanism. Mol Cell Biochem. 2012;364:181-91.
- [CrossRef] [PubMed] [Google Scholar]
- Production of withaferin A in shoot cultures of Withania somnifera. Planta Med. 2001;67:432-6.
- [CrossRef] [PubMed] [Google Scholar]
- The methanolic extract of Withania somnifera ACTS on GABAA receptors in gonadotropin releasing hormone (GnRH) neurons in mice. Phytother Res. 2010;24:1147-50.
- [CrossRef] [PubMed] [Google Scholar]
- Withania somnifera aqueous extract facilitates the expression and release of GnRH: In vitro and in vivo study. Neurochem Int. 2015;89:111-9.
- [CrossRef] [PubMed] [Google Scholar]
- Activation of A-Type g-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16:2872-91.
- [CrossRef] [PubMed] [Google Scholar]
- The role of GABA in the regulation of GnRH neurons. Front Neurosci. 2014;8:387.
- [CrossRef] [PubMed] [Google Scholar]
- Withanone, an active constituent from Withania somnifera affords protection against NMDA-induced excitotoxicity in neuron-like cells. Mol Neurobiol. 2017;54:5061-73.
- [CrossRef] [PubMed] [Google Scholar]
- Ethnological validation of Ashwagandha (Withania somnifera L. Dunal) ghrita as “Vajikarana Rasayana”: In-silico, in-vitro and in-vivo approach. J Ethnopharmacol. 2023;304:116064.
- [CrossRef] [PubMed] [Google Scholar]






