Translate this page into:
Autologous platelet-rich plasma as a potential new approach in the endometrial response during in vitro fertilization cycle: A pilot study

*Corresponding author: Lipi Singh, Saffron Naturele Products Private Ltd., Noida, Uttar Pradesh, India. lipigene@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bakshi R, Kumar U, Prasad B, Gautam SS, Singh L. Autologous platelet-rich plasma as a potential new approach in the endometrial response during in vitro fertilization cycle: A pilot study. J Reprod Healthc Med. 2024;5:3. doi: 10.25259/JRHM_5_2024
Abstract
Objectives:
Infertility is a global health concern, affecting ~13% of couples. Despite assisted reproductive technology (ART) attempts, implantation failure occurs due to inadequate growth of the endometrium. Increasing endometrial thickness (ET) can be increased to improve the rate of pregnancy; implantation necessitates a minimum thickness of 7 mm. Platelet-rich plasma (PRP) is a recommended treatment approach for endometrium and ovarian infertility. This approach minimizes the potential of immunogenic reactions and disease transmission because PRP comes from an autologous source. The purpose of this study was to evaluate the efficacy of PRP intrauterine infusions during the in vitro fertilization (IVF) cycle in patients with thin endometrium.
Material and Methods:
Ten patients with primary infertility (age: 28–40 years) were chosen for intrauterine PRP infusion at the RiSSA IVF Center, Delhi. This study was carried out between June 2020 and January 2022 over a span of 18-month period. Intrauterine infusion of PRP was an additional procedure to hormone replacement therapy (HRT) treatment cycle. PRP was prepared by centrifugation process from autologous blood. On the 10th day of HRT cycle, 2 mL of PRP was infused into the uterine cavity. In each cycle, PRP infusion was administered 1–3 times if there was no increase in ET 72 hours later. Out of ten patients, four patients received a single infusion, two patients received two infusions, and four patients received three infusions. The embryos were transferred when the ET reached ≥7 mm. ET was measured at the uterine longitudinal axis at the thickest point. To determine ET, three measurements were made, and the average of those measures was noted. The primary outcome measure was ET, determined by transvaginal sonography, and the secondary outcome measure was clinical pregnancy following embryo transfer.
Results:
The mean increase in ET was 1.8–2.25 mm. In 8 (80%) patients, there was an increase of 7–7.5 mm in ET. ET thickness did not improve in 2 patients (20%), and it was <6 mm after three infusions of PRP. Further, of the eight patients who had embryo transfer and became pregnant, six patients had a clinical pregnancy with visible cardiac activity at 6 weeks, while two patients had a missed abortion in the first trimester. Six patients had a successful delivery.
Conclusion:
This study revealed that PRP could support endometrial growth, improving pregnancy outcomes in patients who have thin endometrium. PRP is a novel treatment option for endometrial thinning and poor response to IVF. The findings of the current pilot study support the need for large-scale, randomized, controlled trials in this field.
Keywords
Thin endometrium
In vitro fertilization
Platelet-rich plasma
Hormone replacement therapy
INTRODUCTION
Infertility is a global health concern. Approximately 13–15% of couples have trouble conceiving for various reasons associated with implantation failure, quality of the embryo, etc.[1,2] In India, the prevalence of primary infertility is 3.9%–16.8% according to the WHO report.[3] In recent literature, it has been found that the rejuvenation process helps promote tissue regeneration and cure infertility.[4,5]
The uterine cavity, including the endometrium, plays a very important role in ensuring the success of pregnancy. A greater endometrial thickness (ET) is linked to a higher pregnancy rate.[6] While advances in medical technology, such as artificial reproductive technology (ART), have contributed to a notable improvement in pregnancy rates, implantation failure during the in vitro fertilization (IVF) cycle has long been an unresolved issue. Some cycles are terminated during ART because the endometrial development is insufficient.[7] Poor embryo-endometrial contact and endometrial receptivity account for two-thirds of implantation failures, with embryo reasons accounting for one-third of these occurrences.[8] According to recent research, an endometrium that is at least 7 mm thick is necessary for a successful pregnancy.[9] During an IVF cycle, women with thin endometrium have been shown to have a decreased pregnancy rate compared to those with thick endometrial.[10] Therefore, patients with thin endometrium require alternative treatment during the IVF cycle.
The use of growth factors and other cell proliferation techniques to treat and improve endometrium receptivity has been highlighted in recent research as an effective therapeutic option.[11,12] Autologous platelet-rich plasma (PRP) is safe to administer as it reduces the risk of immunological response and infectious diseases as it is drawn from the individual itself.[13] The intrauterine infusion of PRP to enhance endometrial receptivity is grabbing the attention toward ARTs.[14] Autologous PRP is prepared from fresh whole blood, which is enriched with different growth factors such as epidermal growth factor, platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, and other cytokines. These growth factors further promote cell-proliferation, neo-angiogenesis, and anti-inflammatory potential, which have enormous therapeutic implications in tissue engineering and regeneration.[15] Platelet alpha-granules comprise growth factors and cytokines which enable PRP to restore injured tissue by exerting a regenerative effect. These growth factors regulate cell adhesion, migration differentiation and proliferation and promote extracellular matrix accumulation. Calcium ions, dopamine, histamine, serotonin, adenosine diphosphate, and adenosine triphosphate are also found in the dense granules of platelets and are necessary for tissue homeostasis.[16] Additional substances that promote wound healing are found in platelets, including vitronectin, fibronectin, and sphingosine 1-phosphate. The body naturally goes through this healing process on its own.[17] PRP is a useful component for tissue regeneration due to its capacity to initiate the aforementioned process. Therefore, PRP has found extensive application in the therapeutic domain, such as in the promotion of healing in ophthalmology, orthopedics, and healing therapies.[18]
PRP’s regenerative mechanism has been demonstrated to have numerous positive impacts on infertility through clinical trials and animal research.[19-22] Clinical evidence is still preliminary, and little is known about intra-ovarian PRP injection in promoting endometrial proliferation during the IVF cycle. Navali et al. noted that females with poor ovarian response showed better chances of successful pregnancies by a single dose intraovarian injection of autologous PRP.[23] We hypothesized that during IVF, PRP intrauterine infusion can effectively increase ET and improve the outcome of pregnancy. This prospective study aimed at investigating the advantages of PRP for women with thin endometrium who receive embryo transplantation during conventional hormone replacement therapy (HRT).
MATERIAL AND METHODS
Study population and inclusion criteria
Ten patients with primary infertility aged 28–40 years were recruited for intrauterine infusion of PRP at RiSSA IVF Center, Delhi. The study was carried out over a period of 18 months, from June 2020 to November 2021. All patients signed an informed consent. Ten patients who met any of the following inclusion criteria – aged 28–40 years at the time of enrollment, having a history of embryo transfer cancellation due to thin endometrium (<7 mm) in HRT cycles, and having ≥2 unsuccessful IVF cycles – were enrolled in this study. These patients had poor endometrium response to standard HRT (ET <7 mm) on the 13th day of the cycle and were scheduled to receive embryo transfer. Exclusion criteria included chromosomal abnormalities in the patient or spouse, a body mass index (BMI) ≥30 kg/m2, autoimmune disease, and uncontrolled endocrine or other medical disorders (e.g., thyroid diseases).
Study design
| Group/Arm | Treatment |
|---|---|
|
|
PRP: Platelet-rich plasma, ACD-A: Acid citrate dextrose solution A, HRT: Hormone replacement therapy, IVF: In vitro fertilization, ET: Endometrial thickness
ET assessment
The thickest area along the uterine longitudinal axis was used to measure the ET using ultrasonography. To confirm thin endometrium, the ET was measured 2–3 times, and the average of the three readings was noted.
Endometrial preparation
HRT protocol was used to treat each patient. The endometrial priming was started on day 2–3 of the menstrual cycle of the patient, and women were orally administrated with estradiol valerate (E2V) tablets (dose: 6 mg/day); the dose was gradually increased to 12 mg/day according to the endometrium response.[24] Starting on day 6, a transvaginal ultrasound was done every other day to measure ET. Embryo transfer failure occurred in the patient with a non-responsive thin endometrium, resilient to HRT protocol. When ET did not exceed 7 mm, patients were consulted to decide whether to terminate the cycle or undergo PRP treatment. Patients receiving PRP treatment received an intrauterine infusion of PRP on the 10th day when progesterone was administered in the HRT cycle.
Blood collection, PRP preparation, and infusion
The PRP preparation process comprised two centrifugation steps. All steps were performed in a refrigerated centrifuge. On each PRP administration day, 15–18 mL of venous blood was collected from the peripheral vein of the patient using a 20 mL syringe prefilled with 5 mL acid citrate dextrose solution A, an anticoagulant solution A. Subsequently, the blood samples were transferred to aseptic PRP centrifuge kit (N-RP 20, Korea make) and centrifuged for 10 min at 3000 rpm at room temperature (25°C). The blood was divided into three layers- concentrated platelets in the buffy coat layer, cellular plasma in the supernatant, and red blood cells at the bottom [Figure 1]. PRP was extracted from the layer above the buffy coat. Using an Embryo Transfer catheter, 2.0 mL of PRP was administered immediately into the uterus [Figure 2]. ET was reassessed after 48 hours. If the ET was not met, PRP infusion was carried out 2 or 3 more times. Of ten patients, two patients received a single infusion, two patients received two infusions, and the remaining four underwent three infusions [Table 1].

- (a) Preparation of platelet-rich plasma (PRP). (b) Prepared PRP intrauterine infusion within 30 min of its preparation.
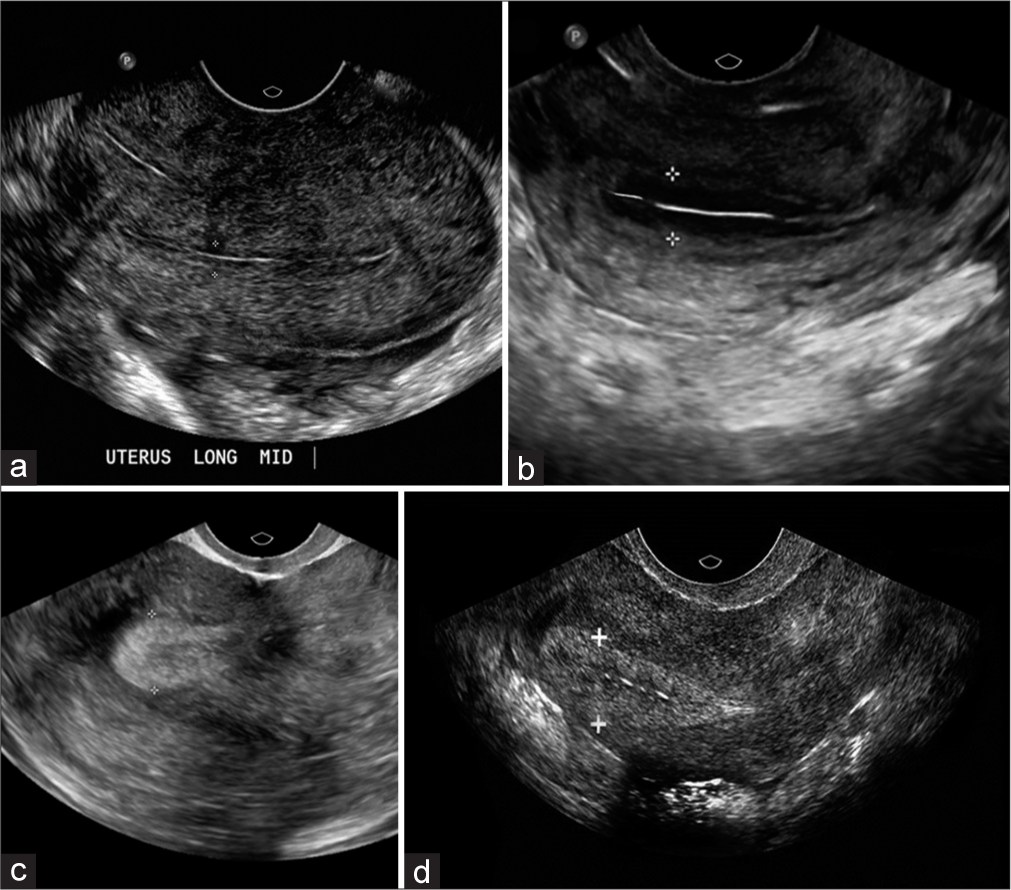
- Ultrasonography in patients during the in vitro fertilization cycle. (a) Thin endometrial thickness (ET) below 6 mm on the day of progesterone administration in conventional treatment/IVF cycle. (b) Improvement in endometrial thickness after first infusion of platelet-rich plasma (PRP) (≥7 mm before progesterone). (c) ET after the second infusion of PRP (≥7 mm before progesterone). (d) Endometrial thickness after the third PRP infusion reached 7 mm on day 5 before progesterone administration.
| Patient number | ET (mm); PRP/48 h after first PRP | ET (mm); 48 h after the second PRP |
ET (mm); 48 h after the third PRP |
Number of embryos transferred | Biochemical pregnancy (beta-hCG) | Clinical pregnancy | Successful pregnancy | Failed IVF cycle |
|---|---|---|---|---|---|---|---|---|
| 1 | <6.8 | 7.2 | _ | 2; Day 05 | Positive | Positive | Yes | 3 |
| 2 | ≥7 | _ | _ | 2; Day 03 | Positive | Positive | Yes | 3 |
| 3 | >6 | 6.3 | 7.4 | 2; Day 05 | Positive | Positive | Yes | 3 |
| 4 | 7.3 | _ | _ | 2; Day 03 | Positive | Positive | Yes | 2 |
| 5 | ≥7 | _ | _ | 2; Day 03 | Positive | Missed Abortion in first trimester | - | 3 |
| 6 | <6.5 | ≥7 | _ | 2; Day 05 | Positive | Positive | Yes | 2 |
| 7 | 7.5 | _ | _ | 2; Day 03 | Positive | Positive | Yes | 3 |
| 8 | <5.8 | >5.8 | >5.8 | _ | Negative | Negative | - | 3 |
| 9 | >6.6 | >6.8 | 7 | 2; Day 05 | Positive | Missed Abortion in first trimester | - | 4 |
| 10 | <5 | >5 | >5 | _ | Negative | Negative | - | 2 |
PRP: Platelet-rich plasma, ET: Endometrial thickness, hCG: Human chorionic gonadotropin, IVF: In vitro fertilization
Embryo transplantation
All the patients were administrated with two good-quality blastocyst-stage embryos. After embryo transplantation, 40 mg of progesterone was injected intramuscularly daily to sustain the luteal phase. The criterion for embryo quality was assessed using the blastocyst scoring system.[25] Serum human chorionic gonadotropin (hCG) level was tested 14 days post embryo transfer. Two weeks later, transvaginal sonography was performed on patients with positive beta-hCG levels to confirm a clinical pregnancy. There were no multiple pregnancies.
Primary outcome
ET: The maximum distance between the two endometrial–myometrial junction surfaces in the midsagittal plane of the uterus was measured using B ultrasound radiography to determine ET.[26]
Adverse reactions.
Secondary outcome
Clinical pregnancy rate: It was defined as the detection, 4–5 weeks after embryo transfer, of a gestational sac with fetal heart activity on ultrasound imaging.
RESULTS
Patient population and baseline characteristics
Twenty patients were assessed for eligibility in the present study. Out of 20 patients, 10 patients who did not meet the inclusion criteria were excluded from the study. The mean age of the patients was 34.7 years. Patients were suffering from primary infertility and, hence, were selected for the intrauterine PRP infusion. The mean number of unsuccessful IVF cycles was 2.8. The mean ET on the previous hCG administration cycle or the final day of estrogen priming was 5.07 mm. Table 2 summarizes the overall characteristics of these individuals, including age, BMI, length of infertility, and etiology of infertility.
| S. No. | Age (years) | BMI (kg/m2) | Duration of infertility (years) | Cause of infertility | ET in prior cycle (mm) |
|---|---|---|---|---|---|
| 1. | 40 | 28.2 | 5 | Tubal Factor/PCOS | 4.8 |
| 2. | 40 | 23.6 | 4 | PCOS | 4.9 |
| 3. | 34 | 20.7 | 2 | PCOS | 5.1 |
| 4. | 28 | 26.0 | 2 | Pelvic factors | 4.9 |
| 5. | 36 | 28.6 | 3.5 | PCOS | 5.2 |
| 6. | 33 | 25.7 | 4 | PCOS | 5.1 |
| 7. | 34 | 20.1 | 3 | Endometriosis | 4.8 |
| 8. | 33 | 19.2 | 4 | Pelvic factor/PCOS | 5.8 |
| 9. | 40 | 25.7 | 5 | Endometriosis/Adenomyosis | 5.1 |
| 10. | 29 | 24.0 | 3 | PCOS | 5.0 |
ET: Endometrial thickness, PCOS: Polycystic ovary syndrome, BMI: Body mass index
ET
In the included patients, there is an increase of 1.8–2.25 mm in ET. ET thickness increased in 8 patients (80%), and it was ≥7–7.5 mm. ET thickness did not improve in 2 patients (20%), and it was <6 mm even after three infusions of PRP [Table 3]. ET in prior cycles is shown in Figure 2a. The patient-wise number of PRP infusions with measurement of ET is presented in Table 1. In this, following cycles monitored to day 15, ET was >7 mm in 4 patients after the first PRP [Figure 2b]. The remaining six patients underwent a second PRP infusion, and ET was measured after 48 h. The ET increased by 2.25 mm after the first PRP injection in four patients. On the second PRP infusion, ET in two patients increased to ≥7 mm [Figure 2c], and the mean increase was 0.35. However, two patients did not show any change in ET. The remaining four patients underwent a third PRP infusion. ET in two patients did not improve and remained <6 mm. The remaining two patients showed an increase in ET to ≥7 mm [Figure 2d].
| ET | Number of Patients (%) |
|---|---|
| ≥7 mm (on the day of progesterone start, embryo transfer done) | 8 (80) |
| <6 mm (no improvement, embryo transfer not done) | 2 (20) |
PRP: Platelet-rich plasma, ET: Endometrial thickness
Progesterone was started on the day the ET reached ≥7 mm. Luteal phase support was given to 8 patients.
Adverse events
PRP infusion was well tolerated by all the patients and there were no adverse effects. There were no documented cases of immunogenic responses in patients.
Clinical pregnancy rate
Among ten patients who underwent embryo transfer, eight of them conceived. Six patients experienced a clinical pregnancy with visible heart activity at 6 weeks [Figure 3], and eight patients experienced a biochemical pregnancy [Table 1]. A missed abortion occurred in the 1st trimester in two patients. Four patients have already delivered, and two pregnancies are in progress.
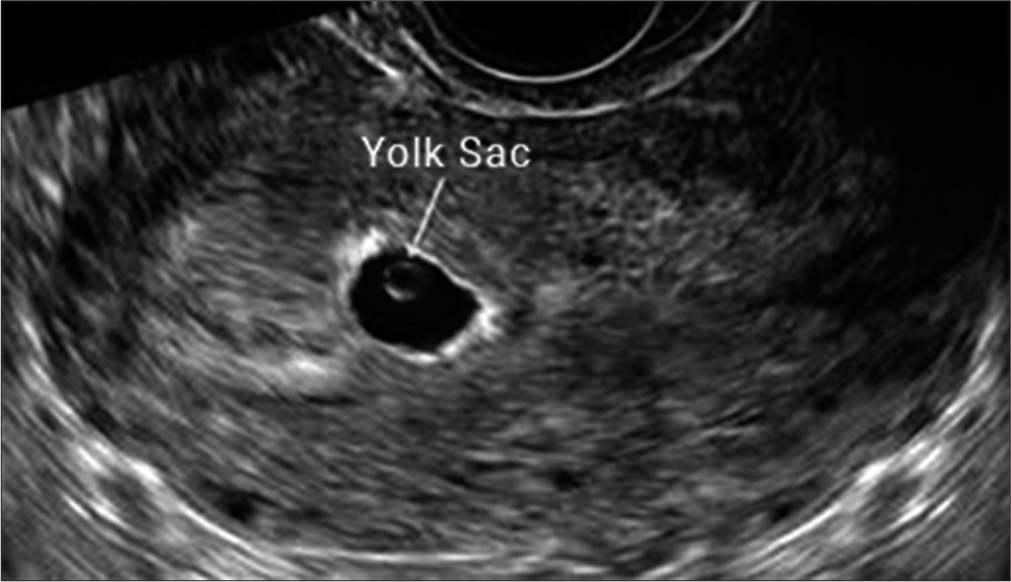
- Intrauterine gestational sac at approximately 6 weeks (transvaginal ultrasonography).
DISCUSSION
The thickness of the endometrium is a significant indicator of reproduction. It is believed that the failure of implantation in the IVF cycle is mainly due to poor receptivity of the uterus.[27] During IVF treatment, the endometrium, controlled by estrogen and progesterone, can be affected by abnormal levels due to ovulation induction, potentially impacting the morphology and receptivity of the endometrium.[28-31] Premature progesterone elevation is caused by high estradiol concentrations during ovarian stimulation, which results in endometrial advancement and hindering implantation.[32,33]
Successful implantation in clinical practice requires adequate endometrial growth, with a minimum thickness of 7 mm needed at the end of the follicular phase for embryo transfer.[30] Exogenous estrogen, electroacupuncture, and the application of granulocyte colony-stimulating factors have been developed as strategies for treating thin endometrium.[34,35] Yet, several patients with thin endometrium remain non-responsive to the above-mentioned remedies. In our study, six patients had poor ET and did not respond to conventional estrogen therapy, resulting in repeated IVF failure (three cycles), low pregnancy potential, and emotional distress.
PRP is a readily obtained, cost-effective, and minimally invasive method. As a patient’s blood is used to attain PRP, it poses minimal risk of infection transmission. Endometrial PRP infusion has been observed to be effective when administered in variable quantities on different days during an HRT cycle.[36] In the current pilot study, we used an aseptic PRP preparation kit with a final platelet count of 717,000–1,565,000/µL and a WBC concentration of 24,000– 37,000/µL, according to the manufacturer. We made an effort to present details on PRP and its preparation process, and we looked for the most well-known data to increase its effectiveness. Using PRP at a platelet concentration of ~1,000,000/µL (503,000–1,729,000/µL) appears to have the optimal biological effect. Higher amounts may have a curiously inhibiting impact, whereas lesser quantities have a suboptimal effect.[37] Following PRP treatment, 80% of patients had adequate ET (>7 mm), and 60% of patients had clinical pregnancy. There are now two active pregnancies and four individuals who have already given birth.
PRP constituents such as cytokines and growth factors work together to prime the endometrium for implantation and pregnancy.[38] Several factors influence endometrial receptivity, one of which is the lack of pathogens that could impede embryo adhesion and implantation. Among IVF patients, chronic endometritis is a very common disease. Unfortunately, endometritis is typically asymptomatic, making diagnosis difficult and treatment ineffectual. Platelet granules include antimicrobial and antibacterial chemicals that can target silent microorganisms that impede embryo implantation. An additional crucial element in endometrial receptivity and implantation of embryos is the expression of adhesion molecules by endometrial cells. For instance, several integrins have a pattern of controlled expression during the menstrual cycle and may be expressed less frequently in infertile women.[39] While not necessarily promoting cell proliferation, the mix of components found in PRP may increase endometrial receptivity by inducing adhesion factor gene expression in endometrial cells. Nevertheless, it is still unclear how precisely PRP components affect the uterine lining. Considering this, the current case series aimed to evaluate the impact of PRP treatment in patients lacking sufficient endometrial lining for the transfer of embryos rather than to regulate or quantify endometrial development.
A thin endometrium is linked to decreased pregnancy rates in IVF procedures. A triple-line pattern and a thickness >7–8 mm on ultrasonography evaluation are requirements for endometria to be considered suitable for embryo transfers.[1,40-42] According to the literature, there is a link between a thin endometrium and poor pregnancy rates.[38] Appropriate endometrial lining contributes to having a higher likelihood of becoming pregnant. Nevertheless, ET is not the only factor at work, especially when considering the results of hormone-stimulated intrauterine insemination (IUI) treatments. Literature suggests no meaningful relationship between ET and IUI pregnancy rates.[43] According to certain reports, embryos created using IUI were less vulnerable to excessive oxygen exposure that happens in thin endometria and more robust in vivo than embryos created through IVF procedures.[43]
The present study is not an analytical cohort study. There was no control group, including patients with inadequate endometrial development who had embryo transfers without PRP injection. Given the various etiologies of infertility, we are unable to draw firm conclusions on how PRP injection improved endometrial receptivity since we lack a comparative group.
In our study, the pregnancy was progressing normally in six women; two women were not responding even after three PRP infusions, and two had missed abortions. On the other hand, miscarriages occurring between weeks 6 and 7 of pregnancy would suggest that the embryos did not survive during or shortly after placenta formation. A significant number of secondary and tertiary villi attached to the maternal decidua should cover the chorionic cavity at the start of the 2nd month of gestation. An embryo-maternal vascular system is born when the capillary system that develops in the middle of the villi rapidly comes into contact with the capillaries of the connecting stalk. The survival of this primitive exchange system is crucial for the embryo to survive through later stages of development. Mishaps at this stage of development could result in the embryo’s death before fetal life. In a comprehensive analysis of women undergoing natural cycle IVF, Wolff et al. did not detect a link between miscarriage rates and ET, supporting the theory that miscarriage may be an implantation process failure irrespective of the influences of embryo quality or ET.[38]
CONCLUSION
Our study demonstrated that intrauterine PRP infusion can enhance ET in women who have a thin endometrium. The majority of patients experienced an increase in ET following PRP infusion, indicating that intrauterine PRP infusion is a novel method for treating thin endometrium that does not respond well to traditional therapy. The results of this investigation support the necessity for future randomized, controlled studies with large sample sizes in this area.
Ethical approval
The research/study is approved by RiSAA IVF Independent Ethics Committee (IEC- RiSAA IVF).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Endometrial thickness, and pregnancy rates after IVF: A systematic review and meta-analysis. Hum Reprod Update. 2014;20:530-41.
- [CrossRef] [PubMed] [Google Scholar]
- A psychological study of male, female related and unexplained infertility in Indian urban couples. J Reprod Infant Psychol. 2017;35:353-64.
- [CrossRef] [PubMed] [Google Scholar]
- A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP) Mol Biol Rep. 2019;46:1611-6.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the effect of platelet-rich plasma on follicular and endometrial growth: A literature review. JBRA Assist Reprod. 2021;25:601-7.
- [CrossRef] [PubMed] [Google Scholar]
- Use of intra-uterine injection of platelet-rich plasma (PRP) for endometrial receptivity and thickness: A literature review of the mechanisms of action. Reprod Sci. 2021;28:1659-70.
- [CrossRef] [PubMed] [Google Scholar]
- Conventional and modern markers of endometrial receptivity: A systematic review and meta-analysis. Hum Reprod Update. 2019;25:202-23.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of vaginal sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil Steril. 2002;78:1073-6.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effect of platelet-rich plasma on experimental ischemia/reperfusion injury in rat ovary. Gynecol Obstet Invest. 2016;81:225-31.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma therapy and reproductive medicine. J Assist Reprod Genet. 2018;35:753-6.
- [CrossRef] [PubMed] [Google Scholar]
- Optimizing platelet-rich plasma gel formation by varying time and gravitational forces during centrifugation. J Oral Implantol. 2013;39:525-32.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of autologous platelet-rich plasma treatment on refractory thin endometrium during the frozen embryo transfer cycle: A pilot study. Front Endocrinol (Lausanne). 2019;10:61.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma as a potential new strategy in the endometrium treatment in Assisted Reproductive Technology. Front Endocrinol (Lausanne). 2021;12:707584.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous platelet-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Medicine (Baltimore). 2019;98:e14062.
- [CrossRef] [PubMed] [Google Scholar]
- Optimized preparation method of platelet-concentrated plasma and noncoagulating platelet-derived factor concentrates maximization of platelet concentration and removal of fibrinogen. Tissue Eng Part C Methods. 2012;18:176-85.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study of 2 methods for obtaining platelet-rich plasma. J Oral Maxillofac Surg. 2007;65:1084-93.
- [CrossRef] [PubMed] [Google Scholar]
- Basic science, and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: A review. Osteoarthritis Cartilage. 2013;21:1627-37.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma injections in acute muscle injury. New Engl J Med. 2014;370:2546-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of autologous platelet-rich plasma on regeneration of damaged endometrium in female rats. Yonsei Med J. 2017;58:1195-203.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma as an adjuvant in the endometrial preparation of patients with refractory endometrium. JBRA Assist Reprod. 2018;22:42-8.
- [CrossRef] [PubMed] [Google Scholar]
- A new approach using autologous platelet-rich plasma (PRP) to treat infertility and to improve population replacement rate. J Res Health Sci. 2016;16:172-3.
- [Google Scholar]
- Successful pregnancy and live birth after intrauterine administration of autologous platelet-rich plasma in a woman with recurrent implantation failure: A case report. Int J Reprod Biomed. 2017;15:803-6.
- [CrossRef] [PubMed] [Google Scholar]
- Intraovarian injection of autologous platelet-rich-plasma improves therapeutic approaches in patients with poor ovarian response: A before-after study. Int J Fertil Sreril. 2022;16:90-4.
- [Google Scholar]
- Cryopreserved embryo transfer: Endometrial preparation and timing. Semin Reprod Med. 2015;33:145-52.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro culture of human blastocyst In: Jansen R, Mortimer D, eds. Towards reproductive certainty infertility and genetics beyond 1999. Carnforth: Parthenon Press; 1999. p. :378-88.
- [Google Scholar]
- Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: A retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Medicine (Baltimore). 2018;97:e9689.
- [CrossRef] [PubMed] [Google Scholar]
- Implantation in assisted reproduction: A look at endometrial receptivity. Reprod Biomed Online. 2013;27:530-8.
- [CrossRef] [PubMed] [Google Scholar]
- Deciphering the crosstalk of implantation: Advances and challenges. Science. 2002;296:2185-8.
- [CrossRef] [PubMed] [Google Scholar]
- Uterine receptivity for blastocyst implantation. Ann N Y Acad Sci. 1988;541:424-43.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of unresponsive thin endometrium. Fertil Steril. 2011;95:2123.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated progesterone during ovarian stimulation for IVF. Reprod Biomed Online. 2012;24:381-8.
- [CrossRef] [PubMed] [Google Scholar]
- Oestrogen and progesterone action on endometrium: A translational approach to understanding endometrial receptivity. Reprod Biomed Online. 2013;27:497-505.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of gonadotrophic stimulation on integrin expression in the endometrium. Hum Reprod. 2002;17:63-8.
- [CrossRef] [PubMed] [Google Scholar]
- Extended estrogen administration for women with thin endometrium in frozen-thawed in-vitro fertilization programs. J Assist Reprod Genet. 2006;23:337-42.
- [CrossRef] [PubMed] [Google Scholar]
- Electroacupuncture reduces uterine artery blood flow impedance in infertile women. Taiwan J Obstet Gynecol. 2009;48:148-51.
- [CrossRef] [PubMed] [Google Scholar]
- Current clinical applications of platelet-rich plasma in various gynecological disorders: An appraisal of theory and practice. Clin Exp Reprod Med. 2018;45:67-74.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34:665-71.
- [CrossRef] [PubMed] [Google Scholar]
- Regulated expression of cytokines in human endometrium throughout the menstrual cycle: Dysregulation in habitual abortion. Mol Hum Reprod. 2000;6:627-34.
- [CrossRef] [PubMed] [Google Scholar]
- Use of integrins to date the endometrium. Fertil Steril. 2000;73:779-87.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of platelet-rich plasma in a model of bovine endometrial inflammation in vitro. Reprod Biol Endocrinol. 2016;14:58.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil Steril. 2007;87:53-9.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reprod Biol Endocrinol. 2012;10:100.
- [CrossRef] [PubMed] [Google Scholar]
- Endometrial thickness in women undergoing IUI with ovarian stimulation, How thick is too thin? A systematic review and meta-analysis. Hum Reprod. 2017;32:1009-18.
- [CrossRef] [PubMed] [Google Scholar]






