Translate this page into:
Spermatozoa HSP90b expression correlates with ROS generation and altered motility in response to methyl parathion treatment in vitro

*Corresponding authors: Luna Samanta, Department of Zoology, Ravenshaw University, Cuttack, Odisha, India. lsamanta@ravenshawuniversity.ac.in
Jasmine Nayak Department of Zoology, Ravenshaw University, Cuttack, Odisha, India. nayakjasmine09@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nayak J, Jena SR, Panda B, Kar S, Samanta L. Spermatozoa HSP90b expression correlates with ROS generation and altered motility in response to methyl parathion treatment in vitro. J Reprod Healthc Med 2022;3:7.
Abstract
Objectives:
Widely used organophosphorus pesticides, methyl parathion (MePa), alter the reproductive functions in various animals and humans by induction of oxidative stress on augmented release of reactive oxygen species (ROS). MePa affects semen quality by inducing DNA damage through spermatogenic stages. Several heat shock proteins (HSPs) are expressed in response to environmental stressors particularly the redox-active ones for regulation of protein turnover. Since oxidative stress and sperm motility are implicated in MePa toxicity, studying the expression of HSP90b will unravel the mechanism behind its noxiousness.
Material and Methods:
Spermatozoa isolated from healthy donors were subjected to various concentrations of MePa (50, 250, 500, and 750 μM) for studying its effect on sperm motility, ROS generation, sperm chromatin integrity, and expression of stress responsive molecular chaperone HSP90b.
Results:
In vitro exposure of MePa at concentrations ≥500 μM results in a decline in sperm motility and an increased generation of ROS, DNA damage, and HSP90b expression.
Conclusion:
ROS-mediated modulation of HSP90b expression may affect the structural integrity of client proteins and oxidative injury to membrane lipid, along with DNA integrity resulting in declined sperm motility in response to MePa.
Keywords
Methyl parathion
Oxidative stress
HSP90b
Spermatozoa
Reactive oxygen species
Motility
INTRODUCTION
Agrochemicals like organophosphorus pesticides (OPs) are used worldwide as pesticides and are hazardous to human health. However, they are mainly known for their neurotoxic effects on non-target species through inhibition of acetylcholinesterase (AChE) activity together with the potential to generate reactive oxygen species (ROS) inducing oxidative stress during their metabolism.[1] Based on this, majority of studies are focused on neurotoxicity of OPs in both non-target and model organisms. The previous studies on methyl parathion (MePa) revealed that it has adverse effects on semen quality in humans and animals.[2,3] Reproductive potential principally in terms of semen quality and hormonal level are reported to be adversely impacted in response to OPs.[4,5] Furthermore, epidemiological studies reveal multifarious reproductive disorders such as infertility and adverse pregnancy outcomes including perinatal mortality and congenital defect as a consequence of OP exposure.[6] The OP pesticides alter reproductive functions primarily through reduction in the activity of brain AChE and, in turn, influence the gonads. Several studies on human exposure to OP pesticide have reported reduction in sperm count and motility, hormonal imbalances such as low testosterone and high luteinizing hormone levels, and increased sex chromosome aneuploidy in sperm.[7] Decline in sperm chromatin integrity is observed in agricultural workers in response to OP exposure particularly MePa, methamidophos, and DZN.[5]
MePa, a toxic organophosphate insecticide has great demand for its broad-spectrum activity. It plays a significant role in impairing sperm function. Changes in the histoarchitecture of testis of adult white throated munias (Lonchura malabarica) are observed on exposure to MePa (5–20 g/100 g body weight/day), where the higher doses reduced the testicular weight after 10 days. On the other hand, a marked decline in seminiferous tubules was evidenced after single administration of lower dose, thus implying impairment of the cholinergic functions of the testes by MePa.[8] A recent study on HepG2 cells exposed to malathion toxicity reported DNA damage and oxidative stress by increase in the level of malondialdehyde (MDA) level, a by-product of lipid peroxidation.[9] Although, Salazar-Arredondo et al. (2008) have reported MePa to decline the motility of human spermatozoa at a concentration of 750 μM, its mechanism of induction is largely unknown.[10] Since oxidative stress and sperm motility are implicated in MePa toxicity, it will not be out of context to hypothesize the heat shock proteins (HSPs) involvement in this process. In fact, the previous proteomic studies by our group have reported the down regulation of different HSPs in human spermatozoa in various male infertility conditions.[11,12] SagarePatil et al. (2017), have also reported a decline in HSP90 in oligoasthenozoospermic men and found a positive correlation with the number of motile spermatozoa. The authors also reported differential expression of HSPs. HSP90a was typically localized in residual nuclear envelope of capacitated human spermatozoa, whereas HSP90b isoform was predominantly flagellar. They also reported that HSP90 inhibition by geldanamycin or 17-AAG was able to suppress progesterone-mediated forward progressive motility, hyperactivation, and acrosome reaction.[13] Under this scenario, the present study is designed to find out the effect of MePa on ROS generation, DNA damage vis-à-vis HSP90b expression, and its correlation with sperm motility in vitro.
MATERIAL AND METHODS
Ethics statement and study population
The proven fertile donors were recruited for the study after the approval by the Institutional Ethics Committee of Kar Clinic and Hospital Pvt. Ltd., Bhubaneswar, Odisha, India.
All donors within reproductive age group 20–35 years having no known medical conditions were included in this study signed an informed written consent.
Semen analysis
The semen samples collected after 3–5 days of sexual abstinence were analyzed following liquefaction for 20– 30 min at 37°C according to the World Health Organization (WHO) 2020 guidelines.[14] Motile spermatozoa fraction was obtained by layering the sample on a double-layer density gradient media (90% and 45% from AllGrad 100%, LifeGlobal) and centrifuging at 2500 rpm for 20 min at 37°C. The pellet containing motile spermatozoa was resuspended in sperm preparation medium (SpermWash, VITROMedia) and subjected to further experimentation.
Spermatozoa treatment with MePa
About 106 motile spermatozoa with approximately 65% motility was incubated with sperm media (1 ml final volume) having different concentrations of MePa (i.e., 50, 250, 500, and 750 μM). Stock solution of MePa was prepared in DMSO. Incubation tube contains spermatozoa and sperm media along with DMSO devoid of MePa served as control. All experiments were replicated 5 times. Sperm motility was reassessed after the completion of incubation period. Since the motility declined significantly at 500 μM MePa, the study of effect of time (0, 15, 30, 45, and 60 min) was carried out by exposing the spermatozoa to 500 μM MePa.
ROS quantification by 2’,7’-dichlorodihydrofluorescein diacetate
The intracellular production of ROS was assessed by oxidation of 2’,7’-dichlorodihydrofluorescein diacetate (H2DCF-DA; Sigma-Aldrich). The non-fluorescent probe, H2DCF-DA on deacetylation by intracellular esterases in presence of ROS, produces highly fluorescent 2,7-dichlorofluorescin. Briefly, spermatozoa (1 × 106/ml) treated with 10 μM H2DCF-DA for 30 min in dark was washed thrice in phosphate buffer saline. Then, the fluorescent signal was detected at 488 nm excitation and 525 nm emission by spectrofluorimetry.
Sperm DNA fragmentation assay
Based on the results of MePa exposure on sperm motility and ROS generation, sperm DNA integrity was assessed after exposure of spermatozoa to 500 μM MePa by Acridine orange (AO; Sigma-Aldrich).[15] AO, a metachromatic fluorescence probe, on binding to double-stranded DNA emit green fluorescence (515–530 nm) while that with single stranded or denatured DNA emits red fluorescence (>630 nm). DNA fragmentation index (DFI) was calculated for quantification of DNA denaturation by the ratio of red to red + green fluorescence and is denoted by proportion of sperm with DFI (DFI%). In brief, smears of the spermatozoa from both control and experimental tubes were air dried, then fixed in Carnoy’s solution (methanol/glacial acetic acid, 3:1) for a minimum of 2 h at 4°C and stained with freshly prepared AO (0.19% w/v in McIlvain phosphate-citrate buffer, pH=4) for 10 min and visualized immediately under fluorescence microscope (Leica, USA) with a 460-nm filter.
Immunocytochemistry (ICC) of expression profile of HSP90β
For ICC, 500 μM MePa (based on results of motility and ROS generation) treated and control spermatozoa were fixed with 4% paraformaldehyde, permeabilized with Triton X-100 (0.1% v/v in PBS) for 5 min and blocked with 1% bovine serum albumin (BSA) (w/v) in phosphate-buffered saline tween (PBST) (0.1% Tween-20) for 2 h. Then, the cells were exposed to primary antibody (HSP90b anti-human goat immunoglobulin [Ig] G, 1:200 dilution; Abcam) in 1% BSA in PBST overnight at 4°C. After washing with PBST, the cells were incubated in FITC conjugated secondary antibody (anti-goat IgG, 1:5000 dilution; Abcam) diluted in blocking solution for 1 h at room temperature in dark. After washing the cells 3 times in PBST, imaging was done on Leica fluorescence microscope (Leica, USA) and the expression of HSP90b was analyzed by measuring the fluorescence intensity using IMAGE J Software.
Statistical analysis
All statistical analysis was done using version 17.8 of MedCalc software (Ostend, Belgium). The data were represented as mean ± Standard deviation. As the data do not follow normal distribution, non-parametric tests were executed for analyses of data on sperm motility and ROS generation (Kruskal–Wallis test), and DFI and time kinetics of sperm motility (Wilcoxon – Mann–Whitney U-test). The expression of HSP90b (fluorescence image intensity) from control and experimental spermatozoa was compared using two-tailed Student’s t-test. Statistical significance was considered if P < 0.05.
RESULTS
Sperm motility, ROS generation, sperm chromatin integrity, and expression of stress responsive molecular chaperone HSP90b were studied after exposure of motile human spermatozoa to different concentrations of MePa.
MePa exposure impaired sperm motility
Lower concentrations of MePa (i.e., 50 and 250 μM) for 60 min at 37°C did not show any effect on motility. However, a significant decrease in motility was observed at 500 and 750 μM MePa [Figure 1]. Since significant decrease in sperm motility was observed at 500 μM, time kinetics of effect of MePa on spermatozoa were studied at this concentration of MePa. When spermatozoa were exposed to 500 μM MePa for different time, their motility significantly decreased at 60 min [Figure 2].

- Effect of different concentrations of methyl parathion (i.e., 50, 250, 500, and 750 μM) at 37oC for 60 min on motility of human spermatozoa. *P < 0.05 with respect to control.
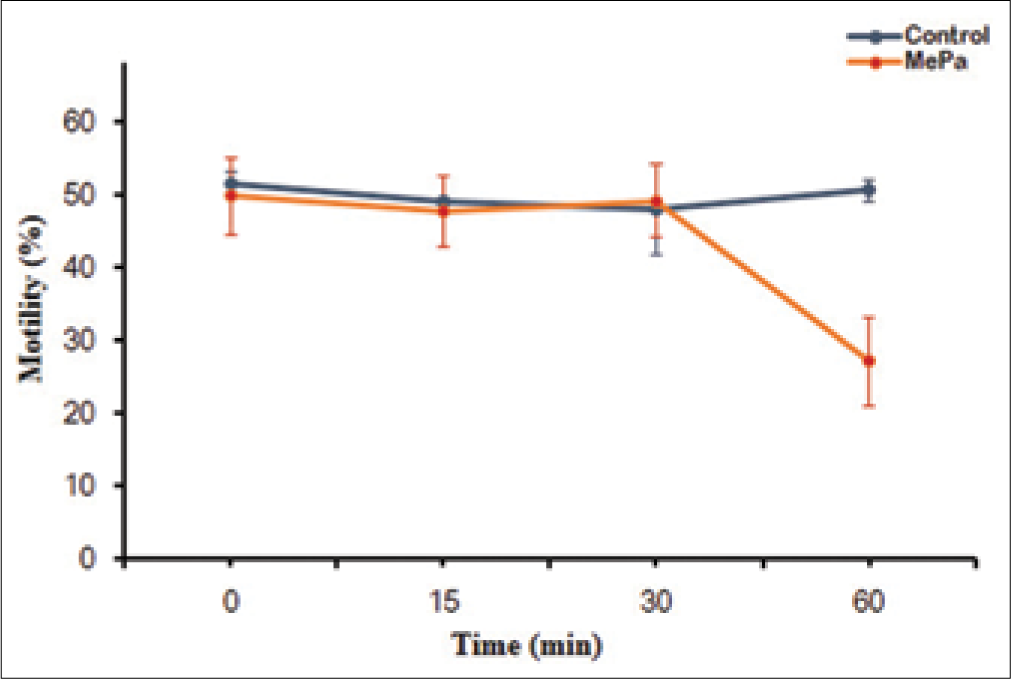
- Effect of exposure time (0, 15, 30, 45 and 60 min) of 500 μM methyl parathion at 37oC for 60 min on motility (%) of human spermatozoa. *P < 0.05 with respect to control.
Effect of MePa on ROS generation by spermatozoa
MePa induces increased generation of ROS in spermatozoa in a dose-dependent manner up to 750 μM [Figure 3] when incubated at 37°C for 60 min. A significant increase in the level of ROS was observed in higher treatment group (i.e., 500 and 750 μM) as compared to control, though no statistical significance was observed between the groups.

- Effect of different concentrations of methyl parathion (i.e., 50, 250, 500, and 750 μM) at 37oC for 60 min on reactive oxygen species generation (H2DCF-DA fluorescence). *P < 0.05 with respect to control.
Alteration in the sperm chromatin integrity by MePa
As significant reduction in motility and augmented generation of ROS was observed in 500 μM treated group, so the effect of MePa on the sperm chromatin was evaluated by DFI at this concentration. It has been observed that MePa-treated spermatozoa have three-fold increase in DFI as compared to control [Figure 4].

- Comparison of DNA fragmentation index between control and 500 μM methyl parathion treated group (Acridine Orange Test). *P < 0.05 with respect to control.
Expression of HSP90β in MePa-treated spermatozoa
HSP90b is found to be primarily expressed in the head and neck region of the spermatozoa and its expression increased significantly when exposed to higher concentration of MePa, that is, 500 μM as evidenced by the difference in intensity of fluorescence measured by fluorescence microscope (Leica, Microscope) [Figure 5].

- Expression profile of heat shock protein HSP90? In control and methyl parathion treated spermatozoa (500 μM) (a); corresponding box-whisker plot of fluorescence intensity of Immunocytochemistry (in arbitrary units) (b). *P < 0.05 with respect to control.
DISCUSSION
OPs, including MePa, were found to be associated with reproductive failures with trans-generational effects. Their genotoxic effects on sperm are opined to be through oxidative damage.[13,16] Pina-Guzman et al. studied on the exposure of MePa on male mice affecting spermatozoa functions decreasing its fertilization ability.[17] Verma et al. (2009), reported that chronic oral toxicity of MePa causes lipid peroxidation in different parts of rat brain (i.e., fore-, mid-, and hind-brain) as evidenced by formation of MDA and 4-hydroxynonanal.[18] In another study, very low doses of MePa (i.e., 0.5–1.5 mg/kg) on rats reported decreased sperm count, along with the formation of abnormal sperms.[4] Similarly, in vitro exposure to MePa is reported to affect DNA integrity in human ejaculated spermatozoa without substantially affecting the motility until 750 μM concentration.[10] Our results corroborated the findings of Salazar-Arredondo et al., with regard to sperm DNA integrity and induction of oxidative stress as revealed by augmented ROS generation. Furthermore, there is a significant decline in sperm motility at 500 and 750 μM MePa exposures with respect to control; although, no marked change was observed between the doses. The generation of ROS is a function of the source of production, for example, number of mitochondria present or number of moles of sperm NADH oxidase. Therefore, maximum rate of generation might have been achieved at 500 μM which did not increase further by augmenting the exposure level to 750 μM. It has been reported that in response to augmented ROS production, transcription and ATP production in mitochondria of boar spermatozoa are severely affected with a damage inflicted to electron transport chain proteins.[19] Therefore, the observed decline in motility might be due to an energy deprived state along with a change in membrane fluidity due to ROS-mediated oxidative damage to the membrane lipids. Interestingly, we observed a sharp increase in HSP90b expression in response to 500 μM MePa exposure. HSPs are constitutively produced molecular chaperones which act in response to several environmental stressors.[20] HSP90b is principally localized in the head (equatorial region). In a recent report, Profumo et al.[21] have shown that induction of oxidative stress by H2O2 upregulated HSP90 on the surface of primary human endothelial cells. Therefore, it is natural to expect an upregulated expression of the chaperone in MePa-induced oxidative stress condition. Furthermore, oxidative damage to HSP90 is shown to cleave the protein into Hsp90cl resulting in degradation of its client proteins.[22] On cleavage, Hsp90cl gains a new function that leads to the accumulation of actin aggregates.[23] Actin polymerizationdepolymerization is suggested to regulate sperm motility including acrosome reaction and capacitation.[24] Therefore, this may also contribute to the decline in sperm motility.
Overall, our study demonstrated that MePa is capable of generating ROS in vitro inflicting damage to the spermatozoa DNA and upregulates HSP90b expression resulting in impaired sperm motility.
CONCLUSION
The organophosphorus pesticides such as methylparathion alter the reproductive functions of animals and humans by inducing oxidative stress through the production of ROS. This ROS mediates the modulation of HSP90β expression which may affect the structural integrity of client proteins and causes oxidative injury to membrane lipids along with DNA integrity resulting in declined sperm motility in response to MePa.
Ethics statement
Institutional Ethics Committee, Kar Clinic and Hospital Pvt. Ltd, Bhubaneswar, Odisha approved the study and all participants gave written informed consent.
Author contributions
LS: Conceptualization. JN and SRJ: Writing-original draft. Methodology: JN, SRJ, BP. LS and JN: Writing-review and editing. The final version of the manuscript is approved by all authors.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Department of Science and Technology (INSPIRE program Grant No. DST/AORC-IF/IF150007); University Grants Commission, Government of India (Grant No. 19/06/2016(i) EU-V); Higher Education Department, Government of Odisha (Grant No 26913/HED/HE-PTC-WB-02-17(OHEPEE).
Conflicts of interest
There are no conflicts of interest.
References
- Biotransformation of organophosphorus compounds. Toxicology. 2001;166:139-60.
- [CrossRef] [Google Scholar]
- Influence of methyl parathion on reproductive parameters in male rats. Environ Toxicol Pharmacol. 2003;14:91-8.
- [CrossRef] [Google Scholar]
- The reproductive toxicity of the organophosphate pesticide 0, 0-dimethyl 0-4-nitrophenyl phosphorothioate (methyl parathion) in the male rat. Folia Morphol (Warsz). 2006;65:309-21.
- [Google Scholar]
- Effects of methyl parathion (o,odimethyl o-4-nitrophenyl phosphorothioate) on rat sperm morphology and sperm count, but not fertility, are associated with decreased ascorbic acid level in the testis. Mutat Res. 2005;588:28-34.
- [CrossRef] [PubMed] [Google Scholar]
- Organophosphorous pesticide exposure alters sperm chromatin structure in Mexican agricultural workers. Toxicol Appl Pharmacol. 2004;196:108-13.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of low-level exposure to organophosphates on human reproduction and survival. Trans R Soc Trop Med Hyg. 2008;102:239-45.
- [CrossRef] [PubMed] [Google Scholar]
- Pesticide interactions and risks of sperm chromosomal abnormalities. Int J Hyg Environ Health. 2019;222:1021-9.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of methyl parathion on gametogenic and acetylcholinesterase activity in the testis of whitethroated munia (Lonchura malabarica) Arch Environ Contam Toxicol. 1996;30:384-9.
- [CrossRef] [PubMed] [Google Scholar]
- Malathion-induced oxidative stress, cytotoxicity, and genotoxicity in human liver carcinoma (HepG2) cells. Environ Toxicol. 2010;25:221-6.
- [CrossRef] [PubMed] [Google Scholar]
- Sperm chromatin alteration and DNA damage by methyl-parathion, chlorpyrifos and diazinon and their oxon metabolites in human spermatozoa. Reprod Toxicol. 2008;25:455-60.
- [CrossRef] [PubMed] [Google Scholar]
- Proteomic signatures of infertile men with clinical varicocele and their validation studies reveal mitochondrial dysfunction leading to infertility. Asian J Androl. 2016;18:282-91.
- [CrossRef] [PubMed] [Google Scholar]
- Proteomic signatures of sperm mitochondria in varicocele: Clinical use as biomarkers of varicocele associated infertility. J Urol. 2018;200:414-22.
- [CrossRef] [PubMed] [Google Scholar]
- Progesterone requires heat shock protein 90 (HSP90) in human sperm to regulate motility and acrosome reaction. J Assist Reprod Genet. 2017;34:495-503.
- [CrossRef] [PubMed] [Google Scholar]
- WHO Laboratory Manual for Examination and Processing of Human Semen (6th ed). Geneva: World Health Organization; 2020.
- [Google Scholar]
- Evaluation of chromatin integrity in human sperm using acridine orange staining with different fixatives and after cryopreservation. Andrologia. 2004;36:321-6.
- [CrossRef] [PubMed] [Google Scholar]
- Methyl parathion causes genetic damage in sperm and disrupts the permeability of the blood-testis barrier by an oxidant mechanism in mice. Toxicology. 2020;438:152463.
- [CrossRef] [PubMed] [Google Scholar]
- Methyl-parathion decreases sperm function and fertilization capacity after targeting spermatocytes and maturing spermatozoa. Toxicol Appl Pharmacol. 2009;238:141-9.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative studies on chlorpyrifos and methyl parathion induced oxidative stress in different parts of rat brain: Attenuation by antioxidant vitamins. Pestic Biochem Physiol. 2009;95:152-8.
- [CrossRef] [Google Scholar]
- Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radic Biol Med. 2019;141:159-71.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding the role of heat shock protein isoforms in male fertility, aging and apoptosis. World J Mens Health. 2014;32:123-32.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative Stress Induces HSP90 upregulation on the surface of primary human endothelial cells: Role of the antioxidant 7,8-dihydroxy-4-methylcoumarin in preventing HSP90 exposure to the immune system. Oxid Med Cell Longev. 2018;2018:2373167.
- [CrossRef] [PubMed] [Google Scholar]
- Hsp90 cleavage by an oxidative stress leads to its client proteins degradation and cancer cell death. Biochem Pharmacol. 2009;77:375-83.
- [CrossRef] [PubMed] [Google Scholar]
- Non-enzymatic cleavage of Hsp90 by oxidative stress leads to actin aggregate formation: A novel gain-of-function mechanism. Redox Biol. 2019;21:101108.
- [CrossRef] [PubMed] [Google Scholar]
- Role of actin cytoskeleton in mammalian sperm capacitation and the acrosome reaction. Reproduction. 2005;129:263-8.
- [CrossRef] [PubMed] [Google Scholar]






