Translate this page into:
Pregnancy management in coronavirus disease – Challenges in developing countries

*Corresponding author: Narendra Malhotra, Department of Obstetrics and Gynecology, Global Rainbow Healthcare, Agra, Uttar Pradesh, India. mnmhagra3@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Malhotra N, Garg R, Singh S, Agrawal P, Malhotra J, Malhotra N. Pregnancy management in coronavirus disease – Challenges in developing countries. J Reprod Healthc Med 2021;2:18.
Abstract
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus (SARS-CoV) infection, first identified in December 2019 in Wuhan, a city in the Hubei Province of China. The infection has spread in more than 150 countries and is a pandemic. Governments across the world have adopted rigorous measures to reduce both the spread by lockdown and cancelling most visas. It has detrimental effects on health-care systems and on the whole economy of world including the USA.
Keywords
SARS-COV-2
COVID-19
Pregnancy with Covid
RTPCR
SOFA
MEWS
ICMR
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus (SARS-CoV) infection, first identified in December 2019 in Wuhan, a city in the Hubei Province of China. The infection has spread in more than 150 countries and is a pandemic. Governments across the world have adopted rigorous measures to reduce both the spread by lockdown and cancelling most visas. It has detrimental effects on health-care systems and on the whole economy of world including the USA.
Pneumonia of COVID-19 is caused by the SARS-CoV-2 a novel enveloped RNA beta coronavirus. It infects host respiratory epithelial cells through angiotensin-converting enzyme 2 (ACE2) – a membrane-bound aminopeptidase which functions as its putative receptor. In India, the first case was reported on January 30, 2020, from Thrissur, Kerala. This index case was originated from Wuhan, China.
What are the symptoms?
The most common symptoms at onset of COVID-19 illness are fever, cough, and fatigue, while other symptoms include sputum production, headache, hemoptysis, rhinorrhea, sore throat, diarrhea, dyspnea, parosmia, anosmia, altered taste and neurological manifestations, and lymphopenia.[1] Clinical features revealed by a chest computed tomography (CT) scan presented as pneumonia, acute respiratory distress syndrome, acute cardiac injury, and incidence of grand-glass opacities that led to death. The symptoms appear after an incubation period of approximately 5.2 days. The period from the onset of COVID-19 symptoms to death ranged from 6 to 41 days with a median of 14 days.[2]
Similarity in symptoms with other beta coronaviruses
Symptoms of COVID-19 and earlier beta coronavirus such as fever, dry cough, dyspnea, lymphopenia, and bilateral ground-glass opacities on chest CT scans are comparable. COVID-19 showed some unique clinical features that include the targeting of the lower airway as evident by upper respiratory tract symptoms such as rhinorrhea, sneezing, and sore throat. Some show an infiltrate in the upper lobe of the lung with increasing dyspnea with hypoxemia. Patients infected with COVID-19 can develop gastrointestinal symptoms like diarrhea, but diarrhea is uncommon in Middle East respiratory syndrome coronavirus (MERS-CoV) or SARS-CoV.[2] Fecal and urine samples should be tested to exclude alternative route of transmission. Case fatality rate of SARS and MERS in pregnancy was very high compared to COVID-19.
DIAGNOSIS
Testing for SARS COV-2
Reverse transcription polymerase chain reaction (RT-PCR) of throat and nasal swab-positive rate bronchoalveolar lavage 95%, sputum 72%, nasal swab 63%, and oropharyngeal swab 32%
Rapid antigen test-immunoglobulin (Ig) M antibodies become positive after the 7th day and IgG on the 14th day
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and value of a CT chest in diagnosing COVID-19 in China are 97%, 25%, 65%, and 83%, respectively (using RT-PCR as a reference)[3]
The teratogenic effects of ionizing radiation on the fetus are inevitable for CT
Exposure to radiation doses <50 mGy is not associated with an increased risk of fetal anomalies or pregnancy loss. The dose of fetal radiation exposure for a routine CT chest is 0.03 mGy. Studies have not demonstrated teratogenicity or thyroid dysfunction in the newborn by intravenous iodinated contrast media (as it crosses the placenta).
In our setup, only RTPCR nasal and throat swabs are allowed.
COVID-19 IN PREGNANCY
Physiological changes in pregnancy encourage rapid progression to respiratory failure in pregnant women. The pregnancy bias toward T-helper 2 (Th2) system dominance which protects the fetus leaves the mother vulnerable to viral infections, which are more effectively contained by the Th1 system [Figure 1].

- Impact of severe acute respiratory syndrome coronavirus 2 on mothers.
SARS-CoV-2 infection during pregnancy is not associated with an increased risk of spontaneous abortion and spontaneous preterm birth. There is no evidence of vertical transmission of SARS-CoV-2 infection when the infection manifests during the third trimester of pregnancy.[4] However, only limited data have been reported till date[6] [Figure 1 and 2].
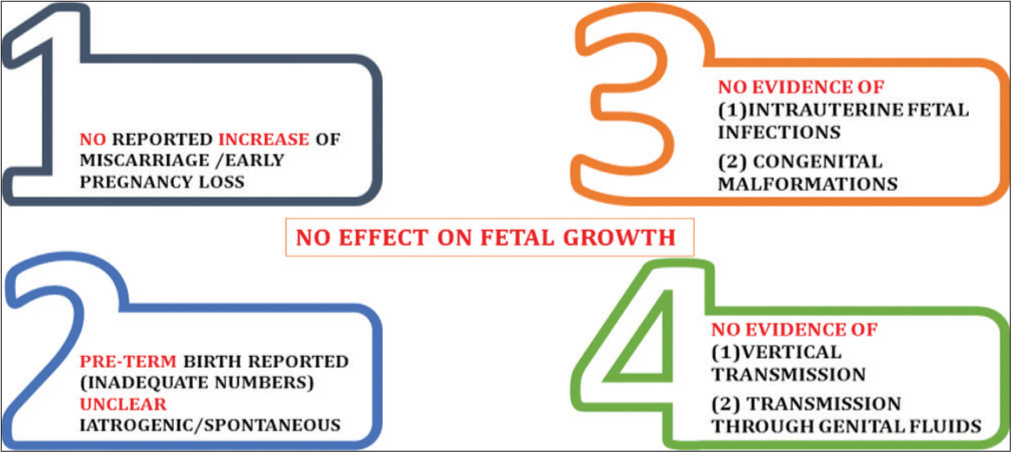
- Effect of severe acute respiratory syndrome coronavirus 2 on fetus.
How COVID infection affects fetus? [Figure 2]
Cohort studies in patients with other infections have not shown increased risks of congenital anomalies from maternal pyrexia in the first trimester but possibility cannot be ruled out. Childhood inattention disorders are more common, due to hyperthermic injury to fetal neurons. Till date, there is no evidence of:
Intrauterine fetal infection,
Congenital malformations,
Effect on fetal growth, and
Transmission through genital fluids.
How mothers are affected in our study at Agra?
Fever, with a median temperature of 38.1–39.0°C, is the prevailing symptom in COVID-19.
More than 50% of hospitalized pregnant women affected by CoV infections have radiological signs of pneumonia, at chest X-ray or computerized tomography and other most common manifestations are cough and lymphopenia [Figure 3].

- Clinical picture of severe acute respiratory syndrome coronavirus 2 in pregnancy.
Pregnancies affected by CoV infections have high rates of preterm birth before 37–34 weeks and miscarriage when the infection is acquired earlier in pregnancy. Miscarriage and preterm birth cannot be attributed solely to COVID since there are no control groups of uninfected women in most studies. Stress in the community might be contributory.
Preeclampsia and cesarean delivery are also more common compared to general population. Perinatal mortality is about 10%, while the most common adverse perinatal outcome is fetal distress, with more than half of the newborn admitted in neonatal intensive care unit (NICU).
According to a retrospective non-randomized review, it is difficult to draw any convincing evidence on this clinical management strategies. This is very short-term follow-up data and thus infections that occurred proximate to the delivery. Risks of pulmonary tuberculosis might be overestimated.
No case of fetal distress, neonatal asphyxia, and admission to NICU was reported. No intrauterine fetal infection occurred as a result of SARS-CoV-2 infection during the third trimester of pregnancy when the time interval from clinical manifestation to delivery was up to 38 days.
Six studies reported information on COVID-19 infection during pregnancy. Miscarriage occurring during the first trimester has not been reported. Preterm birth <37 weeks was approximately 40% and 15% at 34 weeks of gestation, pPROM in 19% while preeclampsia was 13.6% (1/12 95%,CI L2-36.0), with no cases of fetal growth restriction. Cesarean delivery was 91%.[5]
In a small study conducted on women in their third trimester by Wang and Chen who were confirmed to be infected with the coronavirus, there was no evidence that there is transmission from mother to child.[6] However, all pregnant mothers underwent cesarean sections, so it remains unclear whether transmission can occur during vaginal birth. This is important because pregnant mothers are relatively more susceptible to infection by respiratory pathogens and severe pneumonia.
At our center, we have admitted 36 COVID-19-positive pregnancies till now, out of which more than 90% were asymptomatic, 90% were delivered by lower segment cesarean section (LSCS). About 80% elective repeat cesarean were done for 37–39 weeks pregnancy with previous or two LSCS. In all cases, post-operative recovery was uneventful. All babies were negative for COVID 19, enforcing there is no vertical transmission. We have not done testing in amniotic fluid or breast milk. Babies are not breastfed. No case of maternal death has been reported at our institute. It seems infection acquired in the third trimester do not alter maternal and fetal outcomes.
At Rainbow Hospital, Agra, since the COVID disease appearance, 128 deliveries have taken place. As being a tertiary referral care center, the C-section rate is over 50%.
Only RT-PCR negative cases were taken for elective delivery, test done 1 week before date. No COVID positive test case was taken up for delivery as per Government of India guidelines such cases to be referred to COVID hospital. Two COVID-positive antenatal cases were referred. Twenty-five cases emergency C-section was done without COVID test (which was sent just before operation).
However, emergency C-section was done without COVID test in 25 cases with universal precaution and in high-risk OT with all the proper personal protective equipment (PPE). Testing swab for RT-PCR done in all these just prior or at the time of delivery out of 25 asymptomatic non-tested deliveries two came to be +ve postoperatively and was shifted to COVID hospital for L2 case. On follow-up, these were fully recovered and have crossed the 28 days quarantine period also.
Management at COVID hospital, Agra, and Rainbow Hospital, Agra (non-COVID hospital), algorithm for pregnant women with suspected or confirmed COVID-19 infection [Figures 4-7] show the protocols followed at our center for antenatal care.

- Antenatal care for pregnant women with suspected, Probable, or confirmed severe acute respiratory syndrome coronavirus 2 infection.

- Antenatal care for pregnant women with suspected, Probable, or confirmed severe acute respiratory syndrome coronavirus 2 infection.

- Antenatal care for pregnant women with suspected, probable, or confirmed severe acute respiratory syndrome coronavirus 2 infection.

- Pregnancy with suspected severe acute respiratory syndrome coronavirus 2 infection.
First point of contact/initial assessment area for triage and screening.
Any patient with the following symptoms:
Fever ≥38°C [≥100.4 F]
Cough, difficulty in breathing or shortness of breath
Gastrointestinal symptoms.
OR
History of exposure:
Travelled to an affected country within the previous 14 days
Close contact with a confirmed case of COVID-19, that is, <1 m for >15 min, living together, direct contact with body fluids.
Red Tag is given and sampling is done. Give the woman a surgical face mask Maintain minimum 1 m distance from each other Checks for obstetric emergency or labor/delivery issues
Management of suspected cases not in labor
On admission [Figure 8], management depends on severity of disease. Hand hygiene includes use of alcohol-based hand sanitizer that contains 60–95% alcohol before and after all patient contact, contact with potentially infectious material, and before putting on and on removal of PPE, including gloves and washing with soap and water for at least 20 s is also effective. If hands are visibly soiled, use soap and water before use of alcohol-based hand sanitizer.

- Management of confirmed cases symptomatic (severe acute respiratory syndrome coronavirus 2-positive pregnant women).
Institute infection prevention and control measures:
Immediately transfer to an identified isolation room
Donning appropriate PPE by health-care provider.
Testing of the pregnant woman with suspected infection:
Do not delay obstetric care to test for COVID-19
Treat as confirmed COVID-19 until test results are available.
Multidisciplinary approach: By obstetric, neonatal and intensive care specialist. Antenatal care for pregnant woman with suspected or confirmed COVID-19. Assessment is based on clinical picture.
Antenatal contact
Routine appointments (growth scams, oral glucose tolerance test, and antenatal care appointment) delayed until after the recommended period of isolation
No additional tests required
If concerns about the well-being of self or fetus during self-isolation period, patient should be advised to contact maternity team
Provide counseling and information about potential risk of adverse pregnancy outcomes.
Management of confirmed or suspected cases in antenatal care
Hospitalize the pregnant woman based on:
Severity of symptoms
Obstetric emergency/labor.
Maternal early warning criteria [Figure 9]
Systolic blood pressure (BP) <90 or >160 mm of Hg
Diastolic BP 100 mm of Hg
Heart rate <50 or >120/min
Respiratory rate <10 or >30/min
Oxygen saturation in room air of <94
Oliguria defined as urine output
Maternal confusion, agitation, unresponsiveness
Known patient with preeclampsia reporting a nonremitting headache or shortness of breath.

- Triage based assessment of severity: Suspected cases in labor.
Or
Quick sequential organ failure assessment tool–2 out of 3 [Figure 10]
Systolic BP < 100 mmHg
Respiratory rate >22
Altered level of consciousness.
If these criteria are yes – ICU admission, If no – continue monitoring
Severe failure criteria:
(Consider emergency cesarean delivery)
Septic shock
Acute organ failure.

- Maternal early warning criteria, place of admission – assess the need for ICU admission using.
Monitoring of confirmed cases symptomatic
Maternal surveillance
Temperature, IIR, BP, and RR (3–4 times/day)
-
Chest imaging 9 high-resolution CT scan or X-ray
Only if indicated
With abdominal shield
After informed consent.
Consider oxygen therapy to keep O2 saturation >95%
Encourage oral hydration; limit IV fluid if concern for cardiovascular instability.
Monitoring of confirmed cases: Symptomatic
Maternal surveillance: [Figure 11]
Antipyretic therapy
Limit the fetus to the risk of maternal increased body temperature
Screen for other viral infections and/or superimposed bacterial infections
Consider empiric IV/oral antibiotics
Ant malarial/antiviral treatment
Consider thromboprophylaxis.

- Monitoring of confirmed cases (severe acute respiratory syndrome coronavirus 2-positive pregnant women).
Fetal surveillance [Figure 12]
FHR and fetal movement count
Antenatal corticosteroids – for obstetric indications can be given
Women at risk of preterm birth from 24 to 34 weeks of gestation when there is no clinical evidence of maternal infection
Mild COVID-19: Clinical benefits might outweigh the risks of harm to mother.
Antenatal care for pregnant women with suspected or confirmed novel coronavirus. Little evidence of natural history of pregnancy after recovery. Recovery from infection in the 1st trimester: Consider detailed midtrimester anatomy ultrasound examination. Recovery from infection in later half of pregnancy: Consider sonographic assessment of fetal growth 2 weeks after infection
Pregnant women should follow the same recommendations as non-pregnant persons for avoiding exposure to COVID-19 infection
Antenatal care through teleconferencing and video conferencing is the key to providing quality care during pandemic
Triage based on symptom severity and obstetric emergencies [Figure 9]
Multidisciplinary approach to management of suspected or confirmed cases [Figure 10]. MEWS criteria to be adapted for ICU admission.
First and second stage of labor should be managed as in non-COVID cases. Delayed cord clamping has been advocated by some at this stage.[6]

- Fetal surveillance: Symptomatic confirmed cases.
INDIAN COUNCIL OF MEDICAL RESEARCH DEPARTMENT OF HEALTH RESEARCH
Strategy for COVID-19 testing in India (Version 5, dated May 18, 2020)
All symptomatic (influenza-like illness [ILI] symptoms) individuals with a history of international travel in the past 14 days
All symptomatic (ILI symptoms) contacts of laboratory confirmed cases
All symptomatic (ILI symptoms) health care workers/ frontline workers involved in containment and mitigation of COVID-19
All patients of severe acute respiratory infection (SARI)
Asymptomatic direct and high-risk contacts of a confirmed case to be tested once between day 5 and day 10 of coming into contact
All symptomatic ILI within hotspots/containment zones
All hospitalized patients who develop ILI symptoms
All symptomatic ILI among returnees and migrants within 7 days of illness
No emergency procedure (including deliveries) should be delayed for lack of test. However, sample can be sent for testing if indicated as above (1–8), simultaneously.
NB
ILI case is defined as one with acute respiratory infection with fever ≥38°C and cough
SARI case is defined as one with acute respiratory infection with fever ≥ 38°C and cough and requiring hospitalization
All testing in the above categories are recommended by real-time RT-PCR test only
All changes incorporated in these guidelines as compared to the previous version have been indicated in bold.
Indian Council of Medical Research (ICMR) guidelines for obstetric units
The ICMR and the Ministry of Health and Family Welfare have issue the following guidelines [Figures 13-15].

- Latest Indian Council of Medical Research guidelines April 2020.

- Latest Indian Council of Medical Research guidelines April 2020.

- Latest Indian Council of Medical Research guidelines April 2020.
CHALLENGES IN DEVELOPING COUNTRIES
Lack of dedicated COVID hospital beds
Lack of protective equipment for Level 3 care
Lack of standardized N-95 masks
Lack of critical care ICU beds and equipment (Ventilators)
Shortage of workforce – nurses and doctor ratio to population
Overcrowding of hospitals
No clarity on protocols (rapidly changing strategies).
CONCLUSION
COVID-19 has mortality from 3% to 11% in various studies. In the city of Agra with 2.5 million population, case fatality rate of COVID-positive cases is approximately 3.5% with more than 90% mortality due to comorbid conditions. Some viral infections are worse in pregnant women due to physiological changes in pregnancy and relative immunosuppression in pregnancy. However, there is no such documented evidence for coronavirus infections.[5]
Pregnant women are not more susceptible to COVID-19 infection consequences when compared to the general population. Acute respiratory distress syndrome, often requiring invasive ventilation, is the clinical epiphenomenon of the viral pneumonia. Data are changing every day. Hence, we might have to update it soon tell then guidelines to be followed. The challenges in developing countries are little different due to the lack of facilities and over population.
Acknowledgments
We are thankful to the Department of Obstetrics and Gynecology, SN Medical College, for their inputs on managing COVID in pregnancy. We are thankful to Pharmed Ltd. for providing the infographics and figures. We acknowledge the use of I.C.M.R and A.I.I.M.S. COVID management guidelines.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Coronavirus disease 2019 (COVID-19) and pregnancy: Responding to a rapidly evolving situation. Obstet Gynecol. 2020;135:999-1002.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-20.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of chest CT and RT-PCR testing for Coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020;296:E32-40.
- [CrossRef] [PubMed] [Google Scholar]
- Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222:521-31.
- [CrossRef] [PubMed] [Google Scholar]
- Coronavirus disease 2019 in pregnant women: A report based on 116 cases. Am J Obstet Gynecol. 2020;223:111.e1-14.
- [CrossRef] [PubMed] [Google Scholar]
- Updates on COVID-19 infection during pregnancy. Matern Fetal Med. 2020;2:65-7.
- [CrossRef] [Google Scholar]






