Translate this page into:
The genetically engineered vaccine against human chorionic gonadotropin enters Phase I/II clinical trials faces a problem, its possible resolution

-
Received: ,
Accepted: ,
How to cite this article: Talwar GP, Sehgal R, Shrivastava M, Sharma RS, Gupta JC, Gupta SK, et al. The genetically engineered vaccine against human chorionic gonadotropin enters Phase I/II clinical trials faces a problem, its possible resolution. J Reprod Healthc Med 2022;3:2.
Abstract
Objectives:
To determine the causes for formation of nodules in 5 out of 9 women immunized with the genetically engineered vaccine against hCG.
Material and Methods:
Studies were carried out in C57BL/6 strain of mice. Vaccine was given not only by intradermal but also by intramuscular route.
Results:
Nodules were formed in mice when vaccine was given by intradermal route but not by intramuscular route. No antibodies were measurable after 1 µg of the Protein version of the vaccine. However the vaccine given at 2 µg dose (equivalent to 388 µg human dose) induced good antibody response in every mouse.
Conclusion:
Vaccine may be given by intramuscular and not by intradermal route. The dose of the vaccine for women should be at least 300 µg of the Protein version of the vaccine after priming with the DNA version of the vaccine to induce antibody response.
Keywords
hCGβ-LTB
Mycobacterium indicus pranii
Nodules
Resolution
Anti-human chorionic gonadotropin antibodies
INTRODUCTION
A remarkable vaccine was developed against human chorionic gonadotropin (hCG) which was found effective in preventing pregnancy in sexually active women without derangement of ovulation, hormonal profiles, and menstrual regularity.[1] Only one pregnancy occurred in 1224 menstrual cycles. Women regained fertility on the decline of the anti-hCG antibodies and gave birth to normal children.[2]
A genetically engineered version of the vaccine competent to induce the formation of antibodies against hCG was made to enable the production of the vaccine by the industry for availability to public.[3] The vaccine adsorbed on alum along with Mycobacterium indicus pranii (MIP) as adjuvant-induced high titers of anti-hCG antibodies in mice. MIP is an atypical mycobacterium whose genome sequence has been determined.[4] It is a potent invigorator of immune responses. MIP was developed as an immunoprophylactic-cum-therapeutic vaccine for leprosy,[5] but is also effective for curing Category II, difficult to treat tuberculosis patients, clearing of genital warts, and some cancers.[6]
The recombinant vaccine received the approval of the Review Committee of Genetic Manipulations, Department of Biotechnology, Government of India, after extensive toxicology and safety studies. It was then logical to determine whether it prevents pregnancy in women as effectively as the earlier made vaccine based on purified β-hCG from a native source. Permission was obtained from DCGI (Drugs Controller General of India, Government of India) for combined Phase I Safety and Phase II Efficacy trials in women to be conducted at the All India Institute of Medical Sciences (AIIMS), New Delhi, and Sir Ganga Ram Hospital (SGRH), New Delhi.
MATERIAL AND METHODS
Vaccine formulation and immunization schedule
The recombinant hCGβ-LTB vaccine was made both as protein and DNA by M/s Bharat Biotech, Hyderabad, under GMP conditions. Each dose of the DNA version of the vaccine contained also 5 × 108 of heat-killed MIP in a total volume of 1 ml PBS buffer. The protein version of the hCGβ-LTB vaccine was adsorbed on Alhydrogel and contained also 5 × 108 heat-killed MIP per dose. The immunization schedule consisted of delivering two injections of the DNA version of the vaccine (1.5 mg dose) at 15 days intervals followed by one injection of the protein version of the vaccine (100 μg dose).
RESULTS
Observations in clinical trial
Before the COVID-19 pandemic set in, which has kept the trial in suspension for many months, nine women were enrolled in the two centers, three at SGRH, and six at AIIMS.
Table 1 summarizes the observations on the three subjects enrolled and immunized at SGRH.
| Subject | Immunization schedule | Time of nodule formation | Time of nodule resolution |
|---|---|---|---|
| Subject 1 | Two injections of DNA vaccine and one injection of protein vaccine at 15 days interval | Nodule, swelling, and itching at the site on day 3 after protein injection | Follow-up on day 628. Painless nodule still present |
| Subject 2 | Two injections of DNA vaccine and one injection of protein vaccine at 15 days interval | Swelling and itching at the protein injection site on day 3 after protein injection. Nodule also developed at first DNA injection site after 81 days of giving DNA vaccine | Follow-up on day 625. Both nodules disappear |
| Subject 3 | Two injections of DNA vaccine. One injection of protein vaccine could not be given due to the COVID-19 lockdown | Nodule developed after 116 days post-initiation of immunization at the first DNA vaccine injection site | Follow-up on day 543, no pain |
An unexpected observation was the formation of nodules in subjects who received two injections of the DNA version of the vaccine followed by one injection of the protein version of the recombinant vaccine injected with MIP as an adjuvant. In almost all cases, the nodules appeared after the third injection of the protein version of the vaccine. Figure 1 shows the nodules observed in three subjects at Sir Ganga Ram Hospital.
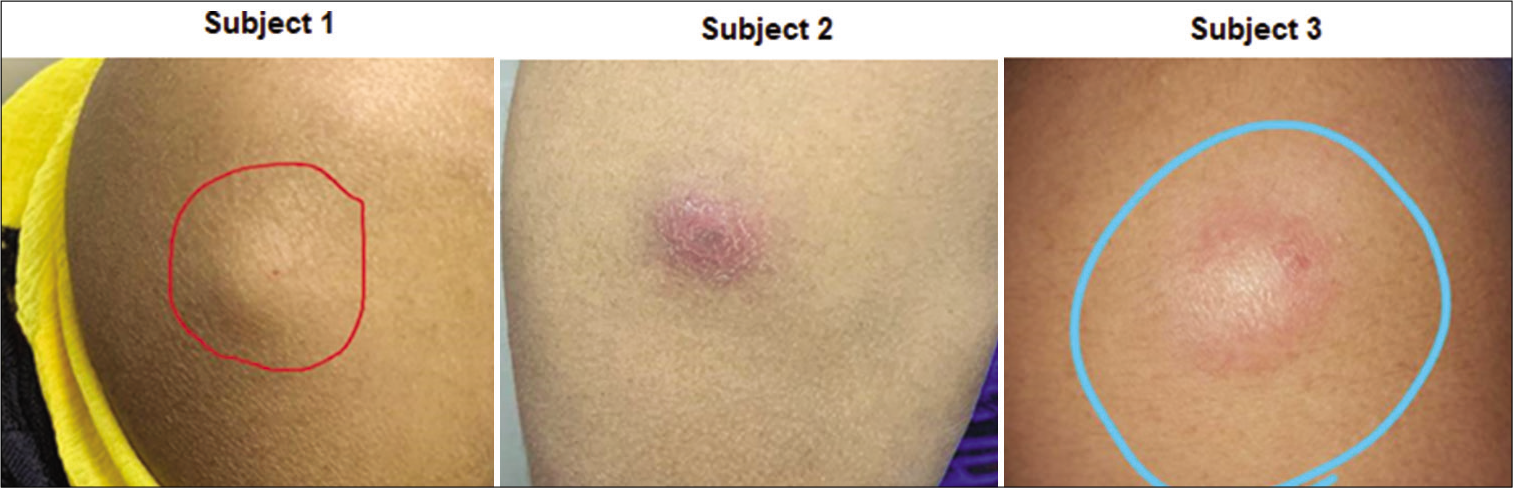
- Nodules formed at the protein injection sites in three subjects enrolled at Sir Ganga Ram Hospital.
At AIIMS, four of the six subjects immunized with the vaccine formed no such nodules. Figure 2 shows the nodules formed in two subjects at the AIIMS center. In course of time, nodules disappeared [Table 2].
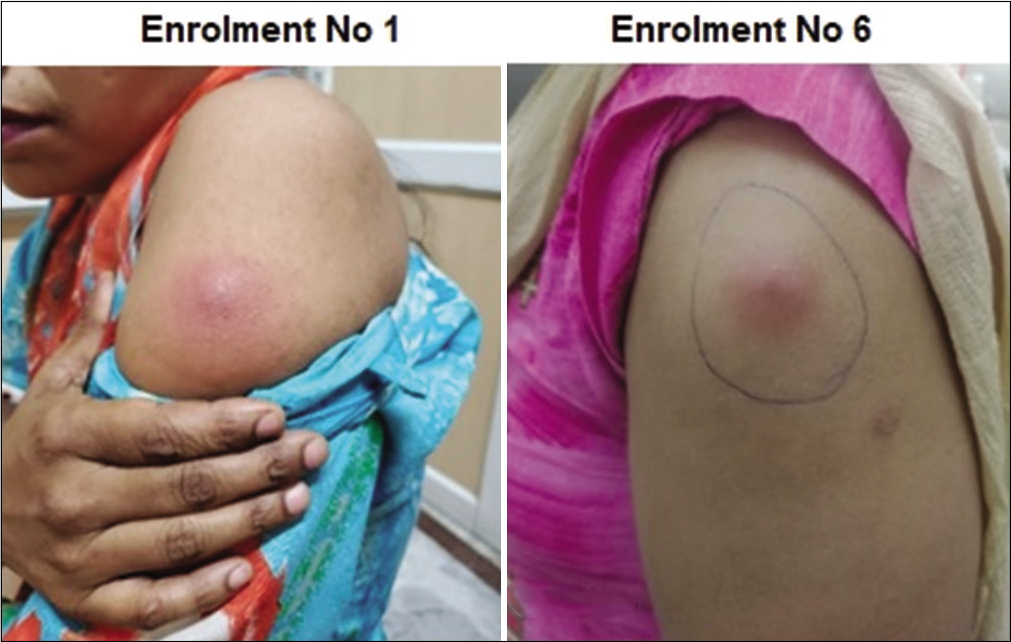
- Nodules formed in two subjects enrolled at the All India Institute of Medical Sciences, New Delhi.
| Subject | Immunization schedule | Time of nodule formation | Time of nodule resolution |
|---|---|---|---|
| Enroll No. 1 | Two injections of DNA vaccine and one injection of protein vaccine | Pain after 2 days at the injection site of protein leading to the formation of abscess on day 11 post protein injection. Incision and drainage had to be done. | Injection site healed by day 60 |
| Enroll No. 2 | Two injections of DNA vaccine and one injection of protein vaccine | No nodule | |
| Enroll No. 3 | Two injections of DNA vaccine and one injection of protein vaccine | No nodule | |
| Enroll No. 4 | Two injections of DNA vaccine and one injection of protein vaccine | No nodule | |
| Enroll No. 5 | Two injections of DNA vaccine and one injection of protein vaccine | No nodule | |
| Enroll No. 6 | Two injections of DNA vaccine and one injection of protein vaccine | Pain at protein as well as first DNA injection sites after giving protein injection leading to the formation of induration and tenderness after 13 days at both test dose and injection sites. An abscess developed after the 22nd day of protein vaccine. | Continued with topical antibiotics from day 119 when she visited the hospital |
Experimental studies in mice
To understand why nodules appeared in some but not all subjects immunized with the vaccine, studies were carried out in female mice of the C57BL/6 strain. The vaccine (priming with DNA version of the vaccine followed by protein as was done in women in a clinical trial) was delivered by two routes in the mice, intradermal and intramuscular at 15 days intervals. Four mice were taken in each group and they received the vaccine at four different doses either intradermally or intramuscularly.
It was observed that nodules appeared in 13 out of 16 mice on delivery of the third and fourth injections, that is, after the first or second protein injection in mice in which the vaccine was given intradermally. This incidentally conforms with the observations in women during the clinical trial, where the nodules appeared after the protein injection. What was remarkable was that 13 out of 16 mice had a nodule on day 35 or day 50, after delivery of the protein vaccine by intradermal route, whereas not a single mouse out of 16 immunized mice were given the vaccine by the intramuscular route had any nodule. These observations are summarized in [Table 3].
| Number of days | Intramuscular | Intradermal |
|---|---|---|
| Day 1 | No nodule | No nodule |
| Day 15 | No nodule | No nodule |
| Day 35 | No nodule | Nodules were observed in 13 out of 16 mice |
| Day 50 | No nodule | Nodules were observed in 13 out of 16 mice |
Antibody response in mice
Studies were carried out in mice to determine whether the genetically engineered vaccine is immunogenic and induces the formation of anti-hCG antibodies. Mice were immunized at two different concentrations of the vaccine, that is, 1 μg and 2 μg dose of the protein version of the vaccine following priming with the DNA version of the vaccine.
It was observed that four injections of the vaccine (two DNA and two protein) given at 1 μg dose generated no antibodies. On the other hand, the same vaccine given at 2 μg dose generated antibodies and these were induced in all four mice immunized with the vaccine, as shown in [Figure 3].
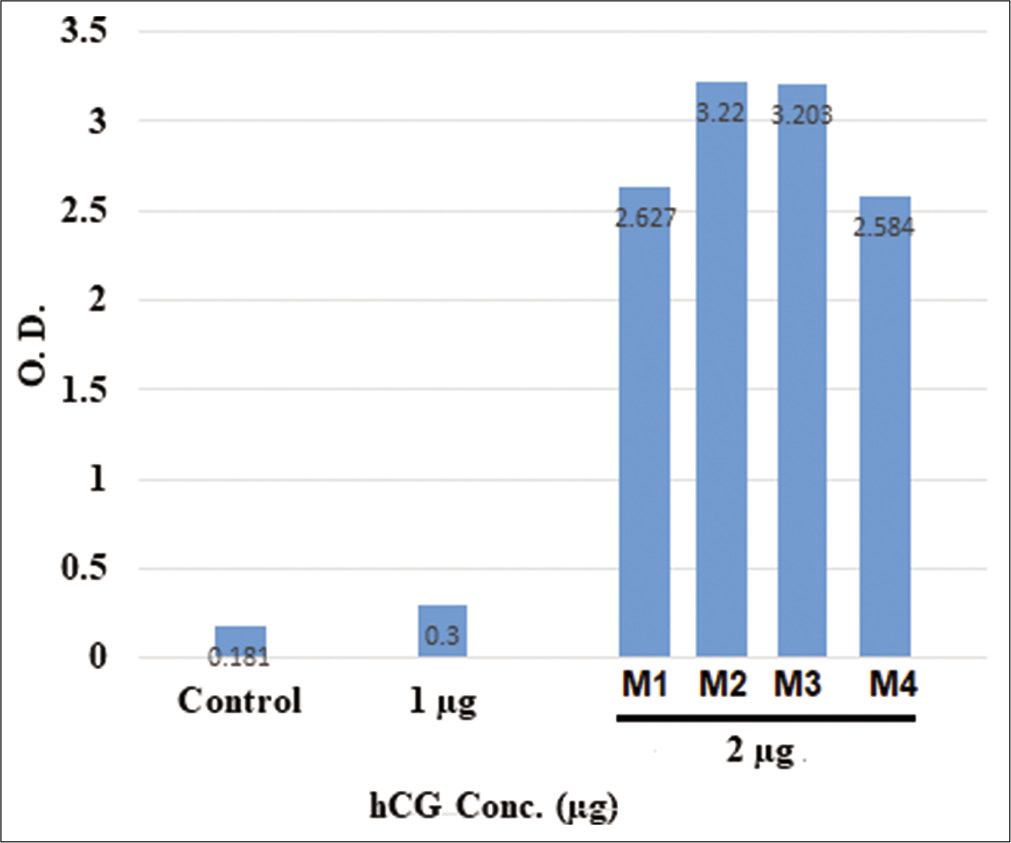
- Generation of antibodies in mice as determined by enzyme-linked immunosorbent assay on day 60 after intramuscular injections of the vaccine when all four injections are given at fortnightly interval. M1, M2, M3, and M4 are four different mice injected with 2 μg of the protein vaccine following priming with DNA version of the vaccine.
It is evident from the figure that every mouse receiving 2 μg dose generated antibodies. The equivalent human dose of 2 μg per mice of 25 g weight is 388 μg for an average human of 60 kg weight.
The protocol for clinical trial envisages the determination of the immunogenicity of the vaccine at various doses. It may be expected from these observations that low doses of the vaccine may not induce sufficient antibodies in women and a dose of 300–400 μg/injection may be necessary to induce the formation of sufficient antibodies.
To sum up, nodules are formed around the injection site in five out of nine women immunized with genetically engineered hCGβ-LTB vaccine adsorbed on alum and administered along with MIP as an adjuvant. Experimental studies in mice indicate that these could be prevented by giving the vaccine intramuscularly instead of intradermally. Furthermore, a dose of 300–400 μg of the vaccine may be necessary for the induction of adequate antibody response.
DISCUSSION
The adjuvant Mycobacterium indicus pranii (MIP) is a mycobacteria. Its repeated administration along with both the 2 injections of the DNA version of the vaccine and Protein version of vaccine may have been the cause of the formation of the Nodule. Experimental studies are required to determine whether the use of MIP in only the first DNA version of the vaccine or twice, along with both the first DNA and the first Protein version of the vaccine, induces sufficient antibody titres.
These studies also indicate that the vaccine should be employed intramuscularly and not intradermally to avoid the formation of Nodules. Furthermore, the doses of the vaccine to induce good antibody response should be 300 to 400 µg of the Protein version of the vaccine.
CONCLUSION
The unexpected formation of Nodules on repeated injections of the genetically engineered vaccine given each time along with the adjuvant MIP, are avoidable by giving the vaccine in each arm 0.5 ml intramuscularly. Observations in mice also indicate that a dose of 300 to 400 µg of the Protein version of the vaccine (equivalent to 2 µg of the mouse dose) should induce good antibody response against hCG.
Acknowledgments
This work was supported by a grant from the Indian Council of Medical Research, New Delhi. We thank Dr. Krishna M. Ella, Managing Director, Bharat Biotech, for making available the vaccine made under GMP conditions free of charge.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
The trial received financial support from the Indian Council of Medical Research.
Conflicts of interest
There are no conflicts of interest.
References
- A vaccine that prevents pregnancy in women. Proc Natl Acad Sci U S A. 1994;91:8532-6.
- [CrossRef] [PubMed] [Google Scholar]
- Regain of fertility and normality of progeny born during below protective threshold antibody titres in women immunized with HSDhCG vaccine. Am J Reprod Immunol. 1998;39:195-8.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a highly immunogenic recombinant candidate vaccine against human chorionic gonadotropin. Vaccine. 2011;29:2341-8.
- [CrossRef] [PubMed] [Google Scholar]
- Polyphasic taxonomic analysis establishes Mycobacterium indicus pranii as a distinct species. PLoS One. 2009;4:e6263.
- [CrossRef] [PubMed] [Google Scholar]
- An immunotherapeutic vaccine for multibacillary leprosy. Int Rev Immunol. 1999;18:229-49.
- [CrossRef] [PubMed] [Google Scholar]
- Besides utility in eradication of leprosy, additional therapeutic benefits of a unique vaccine developed against leprosy. J Mol Biol Ther. 2019;1:6-10.
- [Google Scholar]






