Translate this page into:
Soluble pattern recognizing collectins are integral to human reproductive health

*Corresponding author: Taruna Madan, Department of Development Research, Indian Council of Medical Research Headquarters, New Delhi, India. taruna_m@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Madan T, Gupta HA. Soluble pattern recognizing collectins are integral to human reproductive health. J Reprod Healthc Med. 2025;6:12. doi: 10.25259/JRHM_2_2025
Abstract
Dysregulated immune response and inflammation are characteristic features of ailing reproductive health. Therefore, it is pertinent to decipher the immunology of the female and male reproductive tracts. A series of studies by our group delineated the roles of the three collectins, surfactant protein A (SP-A), surfactant protein D (SP-D), and Mannose-Binding Lectin, integral components of the innate immune response, in reproductive health. The findings demonstrated that gonadal steroid hormones regulated the uterine and testicular expression of SP-D and implicated critical roles of collectins in murine embryo implantation, spermatogenesis, and placental development. The data from clinical studies inferred that SP-D was differentially expressed in the placental tissues of women undergoing spontaneous abortion and women undergoing spontaneous labor suggesting its critical role in regulating inflammation at the feto-maternal interface. In a prospective cohort of 922 pregnant women, serum SP-A, SP-D, and P4/E2 ratio were identified as potential predictive biomarkers of severe Preeclampsia and missed abortion before the 14th week of gestation. Importantly, our collaborative studies established that SP-D reverses the human immunodeficiency virus-1-induced gene expression in an ex vivo model of human vaginal tissue. A direct anti-cancer role of SP-D in both androgen-dependent and independent prostate cancer cells and the tumor biopsies from metastatic prostate cancer patients was reported for the first time. The membrane interactome studies on SP-D identified 347 potential targets on prostate cancer cells including Glucose Regulated Protein of 78 kDa (GRP78), a known prostate cancer cell target. A significantly increased expression of collectins on ectopic mesenchymal stem cells (MSCs) could contribute to endometriotic inflammation. The intensive research has offered the prospect of novel therapies, targeting inflammation-driven anomalous reproductive physiology, to restore immune homeostasis and fertility.
Keywords
Human immunodeficiency virus
Pattern recognition
Pregnancy
Reproductive immunology
Surfactant proteins
INTRODUCTION
Immune cells, besides mounting the systemic host defense against pathogenic organisms, are integral components of human physiological processes in the male and female reproductive tracts such as angiogenesis, tissue remodeling, and induction of apoptosis in persistently activated or abnormal cells, clearance of apoptotic cells, and debris. The gravid uterus and testes, two integral organs to human reproduction, are immune privileged. Their immune privilege status conveys a stringent regulation and modulation of the immune response, a distant fact from the generalized understanding of the absence or downregulation of the immune response. Hence, an interruption in this immune modulation manifests in the form of reproductive disorders. Impairment of spermatogenesis leads to male infertility, whereas, in women, pregnancy complications such as spontaneous miscarriage, recurrent abortion, gestational hypertension, preeclampsia, gestational diabetes, and pre-term labor, are a consequence of an incompetent immune regulation.[1]
The property of pattern recognition by innate immune defense molecules is a remarkably economical skill to eliminate pathogens and abnormal cells. Pattern recognition by collectins, a class of carbohydrate-binding soluble receptors, effectively modulates immune activation, immune suppression, and immunomodulation in human reproductive physiology.
IMMUNOLOGY OF THE HUMAN REPRODUCTIVE TRACT
The human reproductive tract houses an organized and cooperative community of tissue-specific cells and immune cells. The resident leukocytes of the endometrium are actively involved in decidualization, mucosal protection, embryo implantation, and the regulation of tissue breakdown, remodeling, and repair during the menstrual cycle.[2] The uterine natural killer (NK) cells, neutrophils, macrophages, dendritic cells, Tregs, and mast cells have been identified in the endometrium and the fetoplacental unit.[3]
The menstrual cycle alternates between the proliferative phase, secretory phase, and menstrual phase,[4] which is characterized as a controlled “inflammatory” process regulated by stromal cells mimicking leukocytes.[5] Throughout the normal menstrual cycle, the uterine immune cell population and their products undergo substantial changes. The leukocytes, including macrophages, neutrophils, and NK cells, confer protection at the uterine mucosal surfaces in addition to contributing to decidualization, endometrial remodeling and embryo implantation; and in the absence of pregnancy, facilitating the process of menstruation in the absence of implantation.[3,5]
An abnormal shift in the number, distribution, and hence function of the immune cells has been implicated in women with endometriosis and menstrual complaints.[6,7] The resident macrophages within the endometriotic uterus synthesize estradiol promoting the massive proliferation of lesions, along with the production of vascular endothelial growth factor A (VEGF-A), matrix metalloproteinases (MMP), and tumor necrosis factor-alpha (TNF-α) for angiogenesis, tissue degradation, and inflammation, respectively.[8] These changes collectively contribute to the development and activation of local nerve fibers sufficient to trigger pain.[9] Moreover, these alterations in the immune function further usher in implantation failure and infertility.
The products of endometrial immune cells, including inflammatory cytokines and growth factors, play key roles in endometrial physiology and homeostasis. Endometrial VEGF-A, secreted by macrophages, which plays a role in neovascularization, is significantly reduced in women with heavy menstrual bleeding (HMB).[10] Reduced levels of tissue remodeling enzymes, MMP-2 and MMP-9,[11] and significantly elevated levels of pro-inflammatory TNF-α are hallmarks of HMB.[12] Insufficient immune cell activation and exaggerated inflammation in HMB delay regeneration and repair mechanisms in the endometrium. Inflammation facilitates vasodilation, directly disturbing bleeding patterns.[3]
In the male reproductive physiology, active modulation of the immune response confers protection against antigens. During spermatogenesis, the expression of new antigens risks provoking an immune response. These antigens in the seminiferous tubules are spatially isolated from immune cells by the blood-testes barrier. The blood-testes barrier is formed by a layer of Sertoli cells connected by impermeable tight junctions. Nonetheless, the barrier is fragmented areas, such as in the retetestis; wherein other immune mechanisms get involved in the active modulation of pro-inflammatory response.
Testicular immune privilege is, however, temporary, and bacterial/viral infections in the testis can be effectively eliminated. The synthesis of testosterone by Leydig cells may be suppressed by pro-inflammatory cytokines and chemokines through repression of steroidogenic enzymes.[13]
Growing evidence suggests that a local immunosuppressive milieu and systemic immune modulation are critical for maintaining the immune privilege status, based on the investigation of the pattern recognition receptor-mediated innate immune response.[14]
PATTERN RECOGNITION AND IMMUNOMODULATION IN HUMAN REPRODUCTIVE TRACT
Pattern recognition and neutralization are distinct hallmarks of the innate immune system. Collectins are soluble pattern recognition receptors that identify distinct pathogen-associated molecular patterns and thereafter effectively mediate the elimination of the pathogen. Collectins also exhibit immune surveillance properties by discriminating between self and altered self, including tumor cells, necrotic cells, and apoptotic cells. The presence of calcium ions is central to their structural organization, polymerization, and hence their immunomodulatory function.
An acclaimed member of the collectin family is the human surfactant protein D (SP-D), which modulates the immune response, exhibits enhanced anti-viral activity, selectively targets tumor cells, and may potentially function as a diagnostic and predictive marker.[15-17] In addition, studies have indicated that exogenous estrogen and testosterone in female and male mice, respectively, positively regulated SP-D expression, whereas exogenous progesterone negatively regulated SP-D expression.[18] The development of a functional recombinant fragment of human SP-D (rfhSP-D) [Figure 1] has further catapulted the clinical and diagnostic potency of collectins.[19]
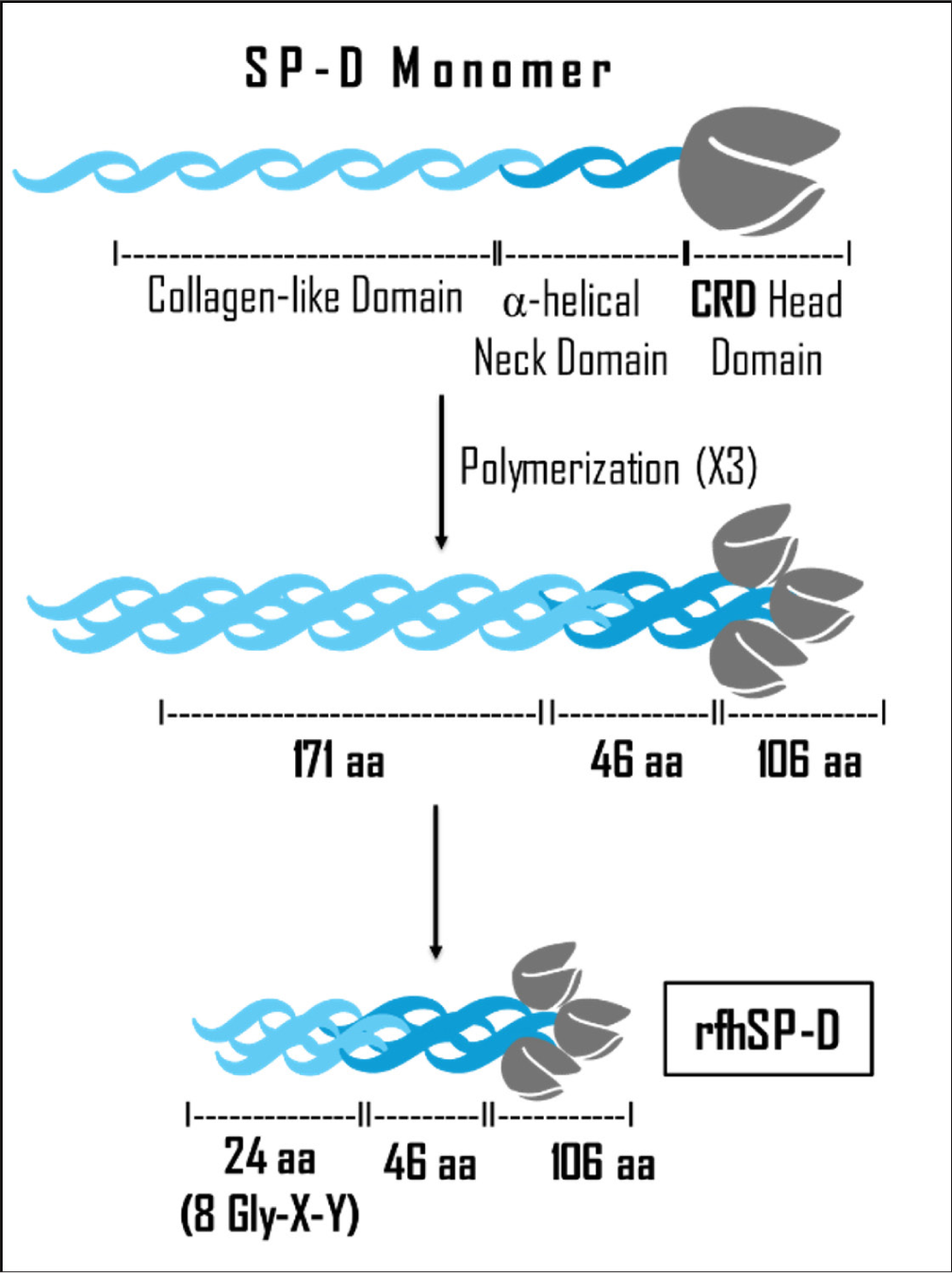
- Structure of rfhSP-D. rfhSP-D: Recombinant fragment of human surfactant protein D. CRD: Carbohydrate recognition domain
The fundamental role of SP-D against human immunodeficiency virus (HIV) was first elucidated with the ability of rfhSP-D to inhibit the replication of HIV-1 binding to viral envelope protein gp120 in a dose-dependent manner. This groundwork also revealed that the introduction of rfhSP-D in HIV-1 challenged peripheral blood mononuclear cells (PBMCs) decreased the levels of pro-inflammatory cytokines.[16] In terms of a cell-mediated immune response, rfhSP-D treatment downregulated the expression of activation receptors, TLR2, TLR4, CD11c, and CD69, in activated PBMCs.[20] rfhSP-D also exhibits protein-protein interaction through its C-type lectin domain with other collectins such as DC-SIGN and represses the transfer of HIV-1 in activated lymphocytes from dendritic cells [Figure 2].[21] Further pre-clinical studies have established that topical application of rfhSP-D (in the form of a vaginal microbicide) impedes the entry of HIV-1 in vaginal cells by reversing HIV-induced gene expression profiles of inflammatory and cytoskeletal proteins; thereby strengthening the vaginal mucosal barrier.[22,23]

- rfhSP-D inhibits the interaction between DC-SIGN and ICAM3, impeding the transfer of HIV-1 from dendritic cells to activated T lymphocytes. rfhSP-D: Recombinant fragment of human surfactant protein D. DC-SIGN: Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin, ICAM3: Intercellular adhesion molecule 3, HIV: Human immunodeficiency virus
The exploration of collectins in embryology and placental biology has revealed that serum collectins [surfactant protein A (SP-A), SP-D, and Mannose-Binding Lectin (MBL)] are constantly downregulated in pregnancy.[15] In the term placenta, SP-D is upregulated, another collectin, SP-A is downregulated, and the labor-inducing pro-inflammatory immune milieu is plausibly controlled by SP-D.[24] A severely low physiological level of collectins in the serum may serve as a potential predictive marker in cases of missed abortion.[25] In other placentopathy such as preeclampsia, serum SP-A and SP-D are remarkably low before the onset of the disease and significantly rise after the onset of the disease. In this prospective study, serum collectin levels were regulated by the estrogen-to-progesterone ratio, which increased after the development of preeclampsia-associated hypertension.[15] The PBMCs harvested from the blood of severe early onset preeclampsia (EOPE) women demonstrated an increase in the expression of the anti-inflammatory M2 phenotype marker, CD163, following rfhSP-D treatment [Figure 3]. This work was ground-breaking in substantiating the use of collectins as predictive and diagnostic biomarkers.
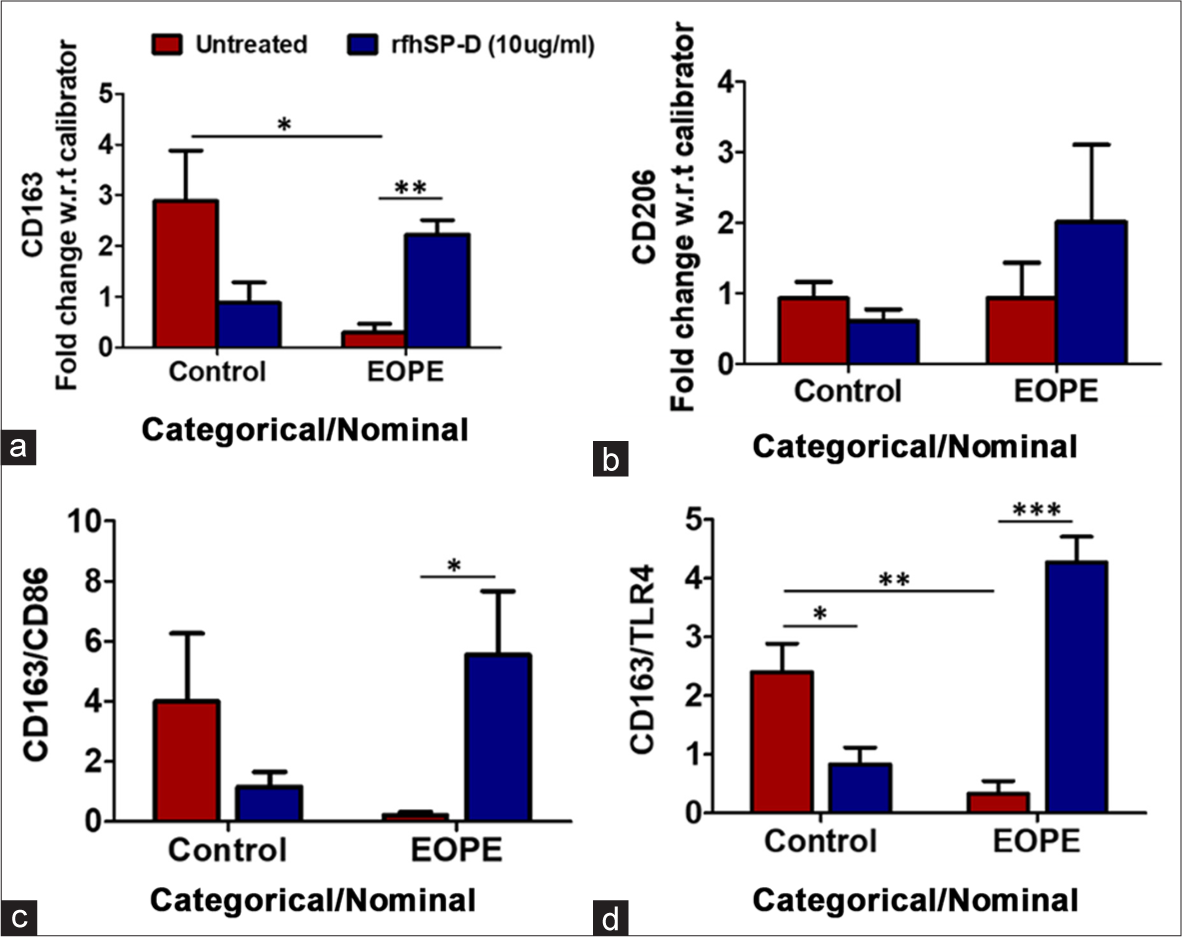
- (a) Fold change in mRNA expression of M2 macrophage marker CD163 in Control Vs EOPE PBMCs treated with rfhSP-D, (b) Fold change in mRNA expression of M2 macrophage marker CD206 in Control Vs EOPE PBMCs treated with rfhSP-D, (c) Ratio of fold change in mRNA expression of CD163 and CD86, co-stimulatory marker of T cell activation, in Control Vs EOPE PBMCs treated with rfhSP-D, (d) Ratio of fold change in mRNA expression of CD163 and TLR4 in Control Vs EOPE PBMCs treated with rfhSP-D. rfhSP-D increased the expression of monocyte M2 phenotype marker CD163 in PBMCs of severe EOPE; Data: Mean ± Standard Error of the Mean; Control (n = 5), EOPE (n = 5),*P < 0.05, **P < 0.01, ***P < 0.001. rfhSP-D: Recombinant fragment of human surfactant protein D, PBMCs: Peripheral blood mononuclear cells, mRNA: Messenger ribonucleic acid.
The contribution of collectins in fertility and reproductive potential has been elucidated from knockout (KO) studies in mice. Female SP-D KO mice demonstrated a dysregulated decidual immune profile and increased preimplantation embryo loss).[26] In addition, pregnant SP-D KO mice develop hypertension and demonstrate a reduced litter size and smaller placentae [Figure 4]. Male SP-D KO mice exhibit enhanced expression of immunosuppressive molecules, serpina 3, transforming growth factor-beta, and interleukin-10 as a compensatory mechanism for SP-D deficiency, highlighting its importance in the maintenance of testicular immune privilege.[27] A spatial expression of collectins was recorded in mice caudal sperm, where SP-A and SP-D localized to the head and tail, whereas MBL localized to the connecting piece.[28]
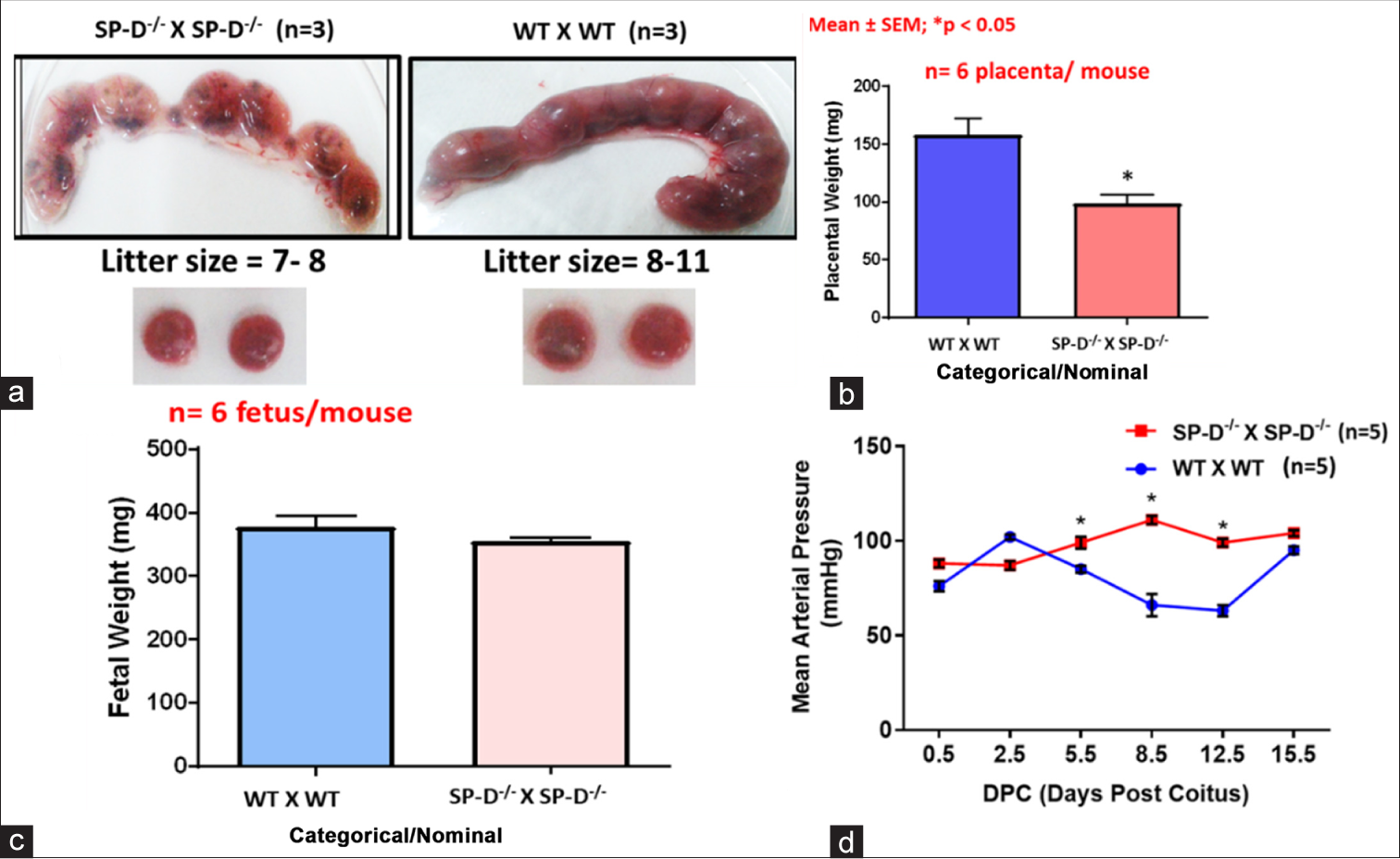
- SP-D KO female mice recapitulate key features of pre-eclampsia during pregnancy, (a and b) significantly decreased litter size and placental weights; (c) low fetal weights; and (d) elevated mean arterial pressure indicative of pregnancy associated hypertension. SP-D: Surfactant protein D, KO: Knockout, WT: Wild type
rfhSP-D has also been evaluated for its anti-cancer activity in prostate cancer. A dose-dependent treatment of rfhSP-D induced selective apoptosis of prostate cancer cells in vitro. This work was further extrapolated to explants from tissue biopsies of metastatic prostate patients, and rfhSP-D-induced apoptosis was evident in this too.[29] A combination of proteomics and in silico studies have revealed GRP78 as a receptor for rfhSP-D, a potential mediator of the rfhSP-D-induced tumor cell apoptosis through p53 and AKT pathways.[17]
Over the past decades, Surfactant Protein D (SP-D) has transitioned from being recognized solely as a pulmonary surfactant-associated protein to an immunomodulatory molecule with systemic relevance [Figure 5].

- Timeline of SP-D emerging as an indispensable innate immune collectin in reproductive health. SP-D: Surfactant protein D.
CONCLUSION
The critical role of SP-D in innate immunity, host-pathogen interactions, metabolic regulation, and reproductive biology has been extensively studied. The generation of recombinant human SP-D (rhSP-D) and rfhSP-D has further expanded our understanding of its functional domains, paving the way for immunotherapeutic and diagnostic applications. From a translational perspective, its role as a biomarker for lung injury, chronic obstructive pulmonary disease, and acute respiratory distress syndrome suggests potential for early disease detection and prognosis. Furthermore, emerging studies on rfhSP-Ds’ anti-cancer and anti-inflammatory properties open new avenues for therapeutic intervention in malignancies and immune-mediated disorders. The ability of SP-D to modulate viral infections, including HIV and influenza, highlights its relevance in antiviral defense strategies. Despite these promising insights, several gaps remain in our understanding of SP-D’s full therapeutic potential. The future research should focus on optimizing rfhSP-D for clinical applications and investigating its role in novel diseases. With advancements in biotechnology and molecular medicine, the next decade holds the potential to translate SP-D research into tangible clinical benefits, further solidifying its role in immunology and disease modulation. Exploring the potential of collectins in biological phenomena and pathological conditions is a treasure yet to be mapped. The diversity of binding ligands and the targeted mechanism of collectins make them extremely versatile effectors of innate immune defense. So far, the collectins continue to restore the “specificity” of the once-called “non-specific” innate immunity.
Acknowledgement:
The presented work has been pursued with the help of several team members and collaborators across the country and beyond borders and we sincerely acknowledge each of the author and the Institutions they are affiliated to, on our publications. We are immensely grateful to all the study participants and experimental animals, Dr. Siddhanath Metkari, Animal House Incharge at NIRRH and his team, the scientific team at the Department of Innate Immunity in NIRRCH and the human contributors to various cells lines that have contributed to this body of research. This wealth of knowledge could be generated only with the generous suggestions from the project selection committees. The various societies that recognized the merit and provided us platforms to seek valuable critique and suggestions from scientific experts are sincerely acknowledged.
Ethical approval:
The information detailed in this review was deduced from studies conducted on human cohorts and animals with the approvals of Institutional Animal Ethics Committee (IAEC) and Institutional Ethics Committee (IEC).
Declaration of patient consent:
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest:
Taruna Madan is on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Department of Biotechnology (DBT), Department of Science & Technology (DST), and Indian Council of Medical Research (ICMR).
References
- The immune privilege of testis and gravid uterus: Same difference? Mol Cell Endocrinol. 2014;382:509-20.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical characterization of stromal leucocytes in nonpregnant human endometrium. Am J Reprod Immunol Microbiol. 1988;17:83-90.
- [CrossRef] [PubMed] [Google Scholar]
- Immunology of normal and abnormal menstruation. Womens Health (Lond). 2013;9:387-95.
- [CrossRef] [PubMed] [Google Scholar]
- Endometrial leukocytes and menstruation. Hum Reprod Update. 2000;6:16-27.
- [CrossRef] [PubMed] [Google Scholar]
- Implantation, menstruation and inflammation. Biol Rev Camb Philos Soc. 1986;61:313-28.
- [CrossRef] [PubMed] [Google Scholar]
- The immunopathophysiology of endometriosis. Trends Mol Med. 2018;24:748-62.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-inflammatory cytokines in endometriosis. Cell Mol Life Sci. 2019;76:2111-32.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology and immune dysfunction in endometriosis. Biomed Res Int. 2015;2015:795976.
- [CrossRef] [PubMed] [Google Scholar]
- New considerations for the pathogenesis of endometriosis. Int J Gynaecol Obstet. 2002;76:117-26.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced levels of VEGF-A and MMP-2 and MMP-9 activity and increased TNF-alpha in menstrual endometrium and effluent in women with menorrhagia. Hum Reprod. 2006;21:2158-66.
- [CrossRef] [PubMed] [Google Scholar]
- Expression and regulation of vascular endothelial growth factor ligands and receptors during menstruation and post-menstrual repair of human endometrium. Mol Hum Reprod. 2006;12:367-75.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor necrosis factor-alpha-associated uterine endothelial injury in vivo Influence of dietary fat. Lab Invest. 1989;61:564-70.
- [Google Scholar]
- Melatonin ameliorates inflammation and oxidative stress by suppressing the p38MAPK signaling pathway in LPS-induced sheep orchitis. Antioxidants (Basel). 2020;9:1277.
- [CrossRef] [PubMed] [Google Scholar]
- Testicular defense systems: Immune privilege and innate immunity. Cell Mol Immunol. 2014;11:428-37.
- [CrossRef] [PubMed] [Google Scholar]
- Differential levels of surfactant protein A, surfactant protein D, and progesterone to estradiol ratio in maternal serum before and after the onset of severe early-onset preeclampsia. Am J Reprod Immunol. 2020;83:e13208.
- [CrossRef] [PubMed] [Google Scholar]
- Surfactant protein D inhibits HIV-1 infection of target cells via interference with gp120-CD4 interaction and modulates pro-inflammatory cytokine production. PLoS One. 2014;9:e102395.
- [CrossRef] [PubMed] [Google Scholar]
- Membrane interactome of a recombinant fragment of human surfactant protein D reveals GRP78 as a novel binding partner in PC3, a metastatic prostate cancer cell line. Front Immunol. 2021;11:600660.
- [CrossRef] [PubMed] [Google Scholar]
- Ovarian hormones regulate SP-D expression in the mouse uterus during estrous cycle and early pregnancy. Am J Reprod Immunol. 2015;74:77-88.
- [CrossRef] [PubMed] [Google Scholar]
- A recombinant fragment of human surfactant protein D binds spike protein and inhibits infectivity and replication of SARS-CoV-2 in clinical samples. Am J Respir Cell Mol Biol. 2021;12:423415.
- [CrossRef] [PubMed] [Google Scholar]
- Surfactant protein D induces immune quiescence and apoptosis of mitogen-activated peripheral blood mononuclear cells. Immunobiology. 2016;221:310-22.
- [CrossRef] [PubMed] [Google Scholar]
- Protein-protein interaction between surfactant protein d and DC-SIGN via c-type lectin domain can suppress HIV-1 transfer. Front Immunol. 2017;8:834.
- [CrossRef] [PubMed] [Google Scholar]
- SP-D impedes transfer of HIV-1 from multi-layered vaginal epithelium with a distinct gene signature. Can J Biotechnol. 2017;1:260.
- [CrossRef] [Google Scholar]
- Surfactant protein D reverses the gene signature of transepithelial HIV-1 passage and restricts the viral transfer across the vaginal barrier. Front Immunol. 2019;10:264.
- [CrossRef] [PubMed] [Google Scholar]
- Differential expression of collectins in human placenta and role in inflammation during spontaneous labor. PLoS One. 2014;9:e108815.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of collectins are sustained during pregnancy: Surfactant protein d levels are dysregulated prior to missed abortion. Reprod Sci. 2020;27:1894-8.
- [CrossRef] [PubMed] [Google Scholar]
- Fertility defects in surfactant associated protein D knockout female mice: Altered ovarian hormone profile. Mol Immunol. 2016;71:87-97.
- [CrossRef] [PubMed] [Google Scholar]
- Surfactant protein D regulates murine testicular immune milieu and sperm functions. Am J Reprod Immunol. 2017;77:e12629.
- [CrossRef] [PubMed] [Google Scholar]
- Testicular expression of SP-A, SP-D and MBL-A is positively regulated by testosterone and modulated by lipopolysaccharide. Immunobiology. 2016;221:975-85.
- [CrossRef] [PubMed] [Google Scholar]
- Human SP-D acts as an innate immune surveillance molecule against androgen-responsive and androgen-resistant prostate cancer cells. Front Oncol. 2019;9:565.
- [CrossRef] [PubMed] [Google Scholar]






