Translate this page into:
RISUG® offers early contraception: An experience during Phase III clinical trials

*Corresponding author: Nirmal Kumar Lohiya, Centre for Advanced Studies, Department of Zoology, University of Rajasthan, Jaipur, Rajasthan, India. lohiyank@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Lohiya NK, Ansari AS, Sadasukhi TC, Pachera S, Khilwani B, Dhaked RK. RISUG® offers early contraception: An experience during Phase III clinical trials. J Reprod Healthc Med 2022;3:11.
Abstract
Objectives:
An early contraceptive efficacy with reasonable assurance of reversibility has been a challenge in male contraception. With nearly four decades of research in reversible inhibition of sperm under guidance (RISUG®) as an intravasal male contraceptive, including pre-clinical trials in rats, rabbits, langur monkeys, and three phases of clinical trials, the present study aims to evaluate the additional parameters of a center of Phase III clinical trials.
Material and Methods:
Subjects were recruited following ICMR guidelines of inclusion and exclusion criteria. Samples were analyzed for sperm functional tests, namely, hypo-osmotic swelling, acrosomal intactness, nuclear chromatin decondensation, and sperm mitochondrial activity index. Furthermore, seminal biochemistry and serum hormones such as follicle-stimulating hormone, luteinizing hormone, testosterone, cortisol, and prolactin were assessed along with levels of anti-sperm antibodies and prostate-specific antigen (PSA).
Results:
The present study, on human subjects, emphasizes the efficacy of RISUG® with early onset of contraception and indication of a greater possibility of reversal. A significant decrease in all sperm functional parameters was observed following RISUG® injection along with increased sperm abnormalities. Semen biochemistry revealed no marked alterations in the concentration of fructose and acid phosphatase, while significantly decreased levels of glycerophosphorylcholine and neutral α-glucosidase were observed. No significant changes in the circulatory levels of hormones and the levels of PSA were observed. In addition, the development of anti-sperm antibodies, an adverse effect of other vas occlusive methods, was not indicated after RISUG® administration, implying the potential of reversibility in humans as observed earlier in different animal models.
Conclusion:
RISUG® presenting deleterious effects on spermatozoa and marked alterations in epididymal markers provides early contraception with a greater possibility of reversal. Although the progress of RISUG® toward development as an ideal male contraceptive is slow, the study implies a strong future possibility.
Keywords
Reversible inhibition of sperm under guidance
Contraception
Reversibility
INTRODUCTION
Annually more than 120 million unintended pregnancies occur worldwide and the contraception is a powerful tool for preventing unwanted pregnancies.[1,2] Globally, there is a plethora of female contraceptives available for use, such as oral contraceptives, diaphragm, and intrauterine devices (IUDs), with high reliability and reversibility. Family planning services have also traditionally targeted women. However, today, many men wish to actively participate in family planning.[3,4] While there are many influential factors, low contraceptive prevalence has been attributed in part to men’s role in family planning.[5]
According to the National Family Health Survey of 2015– 16, only 5–6% of couples use condoms as a method of contraception in India due to high failure rate and also due to the preconceived notion of reduced pleasure.[6,7] Vasectomy, also with the development of no-scalpel vasectomy (NSV), has become a method of choice but is used only by 6–8% of couples worldwide, and < 3% in Asian countries.[8-10] Vasectomy, though considered the most reliable method of male contraception, is not entirely free of impediments. At times, concerns have been expressed that vasectomy may elevate the risk of cancer, cardiovascular, and immune-related diseases.[11] In more than 50% of men, vasectomy leads to an auto-immune response to sperm triggered by phagocytosis in the epididymis.[8] These antibodies increase the probability of infertility after vasectomy reversal.[9,12] Looking toward alternative vas-occlusive contraceptive methods that blocks sperm transport in the vas deferens, various intravasal devices have been examined.[13] These devices and many hormonal formulations have shown contraceptive potential, but none have reached for mass scale application for one or other reason.[14]
Reversible inhibition of sperm under guidance (RISUG®), a copolymer of styrene and maleic anhydride, delivered by no-scalpel injection, offers long-term contraception with safety and efficacy.[15,16] RISUG® injection is a single intervention procedure in which the polymer is injected into vas deferens through a small incision made in the scrotum, thus avoiding surgery during the initial sterilization procedure. Other major advantages of RISUG® include no interruption before the sexual act, cost factor, outpatient procedure, and early azoospermia. Pre-clinical efficacy and safety studies performed on various species of animals including primates were highly successful and followed by efficient Phase-I, II, and III clinical trials on several human volunteers. In a multicentric-limited, Phase III clinical trial administered with a complete dose of RISUG®, Sharma and associates demonstrated oligozoospermia or azoospermia within 2 months of injection.[17] Moreover, RISUG® occlusion has also been proven for its reversal through a non-invasive technique in langur monkeys and functional reversal by dimethyl sulfoxide (DMSO) and sodium bicarbonate (NaHCO3) in rats and rabbits with the establishment of safety up to F1 generation.[15,16] At present, the drug awaits approval from the Drug Controller General of India for mass production.
The presence or absence of spermatozoa should not be the only criteria while considering the fertilizing ability. Occurrence of sperm antibodies, hormone levels in the system, and most importantly functional properties of spermatozoa are significant players in determining both the contraceptive efficacy of a method and also the potential of its successful reversal. Thus, in the present study, toward further exploring the efficacy and safety of RISUG® in human volunteers, semen analysis, sperm functional tests, sperm ultrastructure, circulatory levels of hormones, prostate-specific antigen (PSA), and anti-sperm antibodies were evaluated in a center of Phase III clinical trial.
MATERIAL AND METHODS
Drug
RISUG® is a copolymer synthesized through gamma irradiation of the monomers, styrene, and maleic anhydride, dissolved in the solvent vehicle DMSO in 1:2 ratio. The RISUG® prefilled syringes were kindly supplied by Prof. Sujoy K. Guha, School of Medical Sciences and Technology, Indian Institute of Technology, New Delhi, India.
Subject recruitment
The recruitment of subjects was done by following the recruitment criteria as finalized by the funding agency, namely, age of couple ranged between 25 and 40 years, minimum of two living children (aged >2 years), without pregnancy, no permanent sterilization, and normal reproductive system on clinical examination. The couples were also asked to agree to removal of IUD in case the husband gets enrolled, use of a condom up to 2 months of post-injection or till azoospermia, avoid the use of any contraceptive after 2 months of injection, to undergo medical termination of pregnancy in case of pregnancy during the study period and honor the follow-up schedule for clinical and laboratory examinations. Moreover, couples with sexually transmitted diseases, received treatment for psychiatric/psychological disorders, chronic pelvic infection/cervical carcinoma of wife, diabetes mellitus, cryptorchidism, testicular atrophy etc. of husband, history of vaginitis/cervicitis/cervical dysplasia, and tuberculosis of female reproductive organs/other gynecological problems were excluded from the study.
Based on the above inclusion and exclusion criteria, 28 subjects were enrolled from August 14, 2014, to September 20, 2016. An informed consent form was obtained following the collection of demographic, clinical, and reproductive profiles of the couple. Their chest X-rays, complete blood and urine examinations, liver function tests, kidney function tests, random blood sugar level, and Venereal Disease Research Laboratory tests were carried out. In addition to these tests, scrotal ultrasound examination and ultrasound of lower abdomen and PAP smear test, respectively, of the male subject and his wife were also done. Above examinations and tests were carried out after every 6 months following the RISUG® injection. The study has the approval of the ethics committee, Sawai Man Singh Medical College and Hospital, Jaipur, India.
RISUG® injection
Following pre-injection preparation, the vas was exposed as per the method of NSV and RISUG® was injected (60 mg styrene maleic anhydride dissolved in 120 µL of DMSO) intraluminally with the help of a pre-filled syringe, as per the Standard Operating Procedures as described in the protocol by the Indian Council of Medical Research, New Delhi. Antibiotic augmentin 625 (3 tab/day, GlaxoSmithK Line Pharmaceuticals Ltd., Mumbai, India), tab. chymoral forte (2 × 3 tab/day, Torrent Pharmaceuticals Ltd., Ahmedabad, India), and cap. becosule (1 cap./day, R.N. Pharma, Alwar, India) were prescribed for a period of 7 days following injection. Various instructions such as the use of scrotal support, abstinence for 3 days, and no fluid contact on the scrotal area were also given. Immediate follow-up was scheduled following 3–7 days of RISUG® injection for various clinical examinations such as pyrexia, scrotal inflammation, inflammation of the reproductive system, infection of the urinary system, persistent pain, swelling, sero sangous discharge from the site of injection, lump, nodule, abscess, cyst, urethritis, vesiculitis, cystitis, prostatitis, epididymytis, orchitis, urinary retention, frequency, and testicular atrophy. The above clinical examinations were also carried out at each follow-up visit.
Semen analysis
At least two semen samples, with a minimum of 3 days of abstinence, before the injection procedure were collected which served as reference values for post-injection evaluation. Follow-up samples were collected at 3 weeks, 1.5, 2.5, 4, 5, 6 months, and every 6 months for 5 years post-injection. The samples were collected, after a period of a minimum of 3 days abstinence, by masturbation in a sterile container and kept at 37°C for liquefaction. After liquefaction, semen samples were processed for the following analysis:
Physical characteristics
Semen color, viscosity, pH, liquefaction time, volume, sperm motility, sperm concentration, sperm density, viability, abnormality, agglutination, cellular particulate, germ cells, epithelial cells, leukocytes, and red blood corpuscles were recorded.[18] Sperm morphology was assessed by Papanicolaou’s staining method for counting normal and abnormal sperms.[18]
Sperm functional tests
Sperm functional tests were carried out before and following 21 days, 1.5, 2.5, and 4 months of RISUG injection based on the feasibility of particular tests. A hypo-osmotic swelling (HOS) test that indicates membrane integrity and viability of spermatozoa;[19] Acrosomal intactness (AI) test that indicates the functional status of the sperm acrosome and its ability to penetrate the oocyte;[20] nuclear chromatin decondensation (NCD) test which indicates the ability of the sperm chromatin to undergo decondensation following fertilization,[21] and sperm mitochondrial activity index (SMAI) test which indicates motility and flagellar disorders[22] were carried out at each interval (till azoospermia) using the validated test kits obtained from National Institute of Health and Family Welfare, New Delhi. A score above 60% for HOS and AI tests; above 70% for the NCD test, and above 50% for the SMAI test was considered normal, whereas below these levels were considered to indicate sub-fertility or infertility.[23]
Ultrastructure of spermatozoa
The ultrastructural observations were made before and at every semen analysis interval, before the achievement of azoospermia, sequel to RISUG® injection. Observations on the ultrastructure of spermatozoa were carried out under scanning electron microscopy (SEM).[24] The voided spermatozoa were separated by centrifugation, washed at least thrice with phosphate buffer (0.1 M; pH 7.2) and pelleted by centrifugation. The pellets were immediately fixed in 2.5% glutaraldehyde in phosphate buffer for 30 min and washed thrice in phosphate buffer. A thin film of spermatozoa was smeared on a glass piece, air dried, fixed on an SEM stub with silver paint, dispersed in distilled water, sputter coated with gold, and observed under SEM (Ziess Leo 435 VP, Eindhoven, The Netherlands).[25]
Semen biochemistry
Fructose[26] and acid phosphatase (ACP),[27] (reagent kit, Transasia Biomedicals, Mumbai, India) values were measured in fresh semen samples to determine the functional status of reproductive organs. Seminal plasma free of spermatozoa, obtained after centrifugation, was used for the biochemical analysis of glycerophosphocholine (GPC)[28] and neutral α-glucosidase.[29]
Hormone analysis
Blood sample (2.0 mL) was collected before and at every 6 months of follow-up visit after RISUG® injection by venipuncture in a clean sterile tube and allowed to clot at room temperature. Serum levels of testosterone, follicle stimulating hormone (FSH), luteinizing hormone (LH), cortisol, and prolactin were assayed with commercially available enzyme-linked immunosorbent assay (ELISA) kits (United Biochemical Research Inc., Washington, USA). The normal range as reported by ELISA kits manufacturing company for testosterone, FSH, LH, cortisol, and prolactin, respectively, was 10.41–34.70 nmol/L, 1.0–12.1 mlU/mL, 1.0–12.5 mlU/mL, 50–230 ng/mL, and 2–17 ng/mL.
PSA levels
The stored serum was also assayed for PSA with a commercially available ELISA kit (United Biochemical Research Inc., Washington, USA). The normal value for PSA was <4 ng/mL.
Antisperm antibody (ASA)
The ELISA kit from BIOSERV Diagnostics (BS-10-20) was used for quantitative determination of ASA in serum obtained from all subjects before and after RISUG® injection. The normal range for ASA was 0–60 U/mL.
Statistical analysis
ANOVA was employed for statistical comparison. The difference between means was calculated by Hols-Sidak multiple comparison test to detect the difference using the statistical software (SigmaPlot version 14). The values are expressed as mean ± standard error (SE) and P < 0.05 level was considered significant.
RESULTS
Follow-up studies after RISUG® injection indicated normal health in all male volunteers and their partners with no significant adverse effects. However, most of the subjects showed temporary scrotal enlargement and moderate pain in the scrotum and inguinal region that got resolved without impairment of any routine activity. Following the RISUG® injection, pregnancy did not occur in any of the participating subjects.
Semen analysis
Twenty-eight subjects enrolled in the present study were given RISUG® injection and semen samples were collected during pre-injection followed by various post-injection durations from 21 days up to 5 years. In all subjects, volume, ejaculation time, color, consistency, and pH of semen did not show any appreciable change during the follow-up period in comparison to the pre-injection samples. Motility, viability, and density showed a decline and sperm morphological abnormalities elevated after RISUG® injection and during follow-up. After RISUG® injection, sperm concentration was decreased to lower than 20 million/mL in the first few ejaculates and continued to azoospermia in most of the subjects. The mean sperm count was decreased to the range of 4.4–0.03 million sperm/mL in all subjects during the follow-up duration of 5 years as indicated in [Figure 1]. The onset of azoospermia varied in subjects according to their physiology. In seven subjects out of 28, the early onset of azoospermia was noticed on the 21st day of injection. Similarly, 13, 4, 1, 2, and 1 subjects depicted azoospermia, respectively, at 1.5, 2.5, 4, 5, and 6 months of post-injection. Hence, the earliest azoospermia was observed after 21 days of RISUG® injection which continued till a follow-up duration of 5 years.
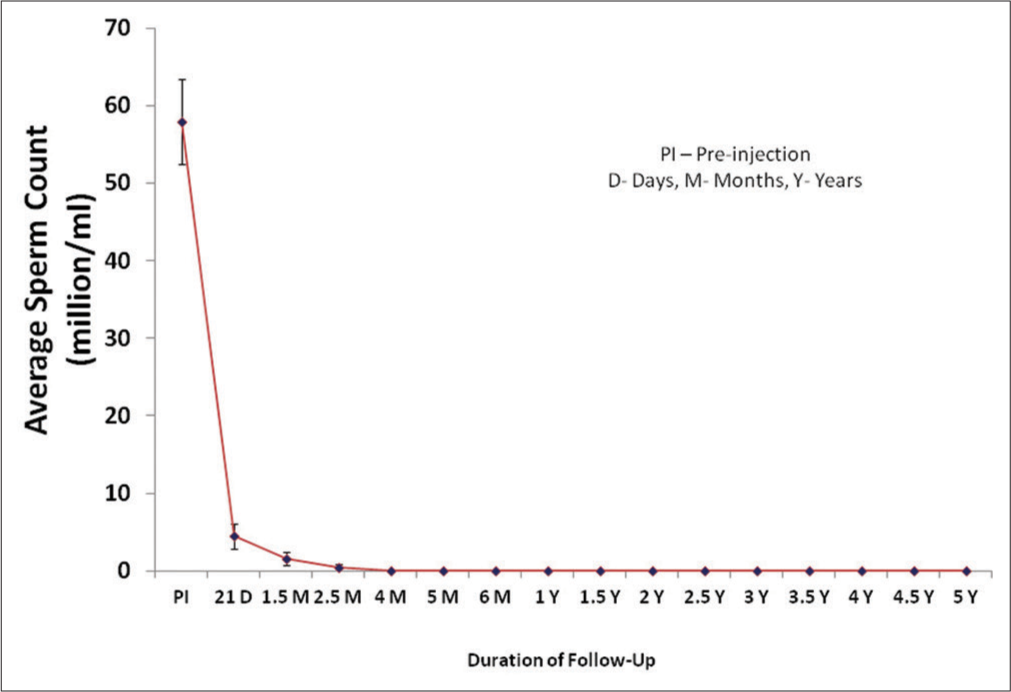
- Mean sperm count of subjects before and following different intervals of reversible inhibition of sperm under guidance injection.
Sperm functional tests
The basic semen analysis has limited predictive value for conception, thus there is a requirement for extended sperm functional testing. Sperm functional tests aim to evaluate basic and significant steps of fertilization such as sperm binding to zona pellucida (ZP), acrosomal exocytosis, and fusion with the vitelline membrane of the oocyte. Before RISUG® injection, functional parameters were evaluated in all subjects under study and all test values showed uniform fertile score that ranged between 62.00% and 89.48% of HOS, 62.00% and 82.15% of AI, 72.00% and 88.54% of NCD, and 51.00% and 71.00% of SMAI, respectively. After RISUG® injection, low sperm functional tests were observed in voided sperms being indicated in the representative figure [Figure 2]. All the sperm functional tests before azoospermia scored in the sub-fertile to sterile range indicating a gradual decrement in the score in the subsequent ejaculates. The range of HOS (25.57–41.67%), AI (32.09–44.59%), NCD (29.99–44.72%), and SMAI test (21.02–32.23%) after RISUG® injection observed below the normal values signifies lack of the fertilization potential of ejaculated sperms [Figure 3].

- Sperm functional tests: Hypo-osmotic swelling test, Acrosomal intactness test, Nuclear chromatin decondensation test, and Sperm mitochondrial activity index test, in voided sperms before azoopsermia, after reversible inhibition of sperm under guidance injection. Representative figures of morphological changes in human spermatozoa. (a) Pre-injection, (b) 21-day

- Sperm functional tests in voided sperms before azoopsermia, after reversible inhibition of sperm under guidance injection. Schematic representation of average values observed for (a) Hypo-osmotic swelling test, (b) Acrosomal intactness test, (c) Nuclear chromatin decondensation test, and (d) Sperm mitochondrial activity index test.
Ultrastructure of spermatozoa
The ultrastructure of ejaculated sperms during pre-injection in all subjects exhibited normal morphology with round head, distinct neck, slender mid-piece, long tail region, and intact cell membrane [Figure 4]. After RISUG® injection before azoospermia (21 days–6 months), various deformities, namely, bent mid-piece/tail, double head/tail, damaged/ disrupted membrane, cytoplasmic droplets, and damaged acrosome, were observed in the ejaculated spermatozoa [Figure 4].

- Ultrastructure scanning electron microscopy of spermatozoa from subjects before and after receiving reversible inhibition of sperm under guidance (RISUG®) injection. (a) Image represents normal structure of spermatozoa before RISUG injection and various defects observed after RISUG injection, (b) coiled tail, (c) coiled tail, bent mid piece, cytoplasmic droplets, (d) double heads, double tails, ruptured acrosome, bent, and ruptured midpiece, (e) coiled tail, (f) cytoplasmic droplets, head-tail separation, (g) coiled tail, (h) damaged membrane, deformed head, cytoplasmic droplets, and (i) damaged acrosome and membrane, bulging nuclear material.
Seminal plasma biochemistry
The biochemical composition of the seminal plasma is extremely complex and differs remarkably in many respects from that of blood plasma and other body fluids. Analysis of seminal biochemical markers in all the subjects before and after RISUG® injection illustrated that seminal vesicle secretion (fructose) and prostatic marker (acid phosphatase) remained unchanged. Following RISUG® injection, the concentration of fructose and activity of acid phosphatase varied only from 15.57 ± 3.62 to 21.13 ± 7.86 µmole/ejaculate and 218.07 ± 27.91 units/ejaculate up to 259.39 ± 50.30 units/ ejaculate, respectively, during the entire period of study [Figure 5a and b]. Neutral α-glucosidase activity and GPC concentrations suggested as seminal markers of epididymal patency were found to decrease significantly following RISUG® injection. Following 5 years of RISUG® injection, the neutral α-glucosidase activity was 7.13 ± 3.27 mU/mL in comparison to pre-injection was 18.36 ± 9.63 mU/ml [Figure 5c]. Similarly, values of GPC decreased from 6.11 ± 0.61 mg/mL during pre-injection to 1.44 ± 0.50 mg/mL following 5 years of RISUG® injection [Figure 5d].

- Mean values of different biochemical parameters before and following different intervals of reversible inhibition of sperm under guidance injection. (a) Fructose, (b) acid phosphatase, (c) neutral α-glucosidase, and (d) glycerophosphorylcholine.
Hormonal analysis
Hormones play a key role in initiating and maintaining the male reproductive function, variability in the levels of hormones such as testosterone, FSH, and LH impact semen quality. After RISUG® injection, serum testosterone levels indicated a slight change in the range between 10.90 ± 2.57 nmol/L and 19.89 ± 8.34 nmol/L as compared to pre-treatment 15.11 ± 7.95 nmol/L, but remained in a normal range of pre-injection [Figure 6a]. Circulatory levels of FSH were 4.69 ± 0.95 mlU/mL to 6.52 ± 3.01 mlU/mL during 5 years after RISUG® injection [Figure 6b]. Similarly, levels of LH, cortisol and prolactin ranged from 5.04 ± 3.18 mlU/mL to 9.37 ± 2.00 mlU/mL, from 83.81 ± 24.49 ng/mL to 121.26 ± 43.59 ng/mL, and 9.43 ± 2.03 ng/mL to 14.45 ± 13.54 ng/mL, respectively, during follow-up duration of 5 years after RISUG® injection [Figure 6c-e]. Levels of all hormones were observed within the range of normal fertile male.

- Mean serum hormone levels before and following different intervals of reversible inhibition of sperm under guidance injection. (a) Testosterone, (b) Follicle Stimulating Hormone, (c) Luteinizing Hormone, (d) Cortisol, and (e) Prolactin.
PSA levels
Serum PSA levels did not change to any significant level following RISUG® injection (0.50 ± 0.38 ng/mL to 0.86 ± 0.59 ng/mL) throughout the maximum follow-up period, that is, 5 years as compared to pre-injection (0.61 ± 0.35 ng/mL) [Figure 7].

- Mean prostate-specific antigen levels of human subjects before and following different intervals of reversible inhibition of sperm under guidance injection.
ASA
The ASAs are composed of numerous antibodies interacting with multiple sperm antigens that play a role in fertility. A few subjects showed the presence of ASA during the pre-injection phase but the values were found to be within the normal range (0–60 U/mL). Sperm antibody titers in the serum of subjects after RISUG® injection fluctuated in the range between 25.70 ± 8.93 U/mL and 35.96 ± 6.67 U/mL and did not show statistically significant changes compared with those of pre-treatment levels (25.63 ± 9.59 U/mL) until 5 years of vas occlusion [Figure 8].

- Mean anti-sperm antibody levels of human subjects before and following different intervals of reversible inhibition of sperm under guidance injection.
DISCUSSION
Most potential users of a male contraceptive expect a method that is safe, effective, reversible, and affordable. Among other vas-based methods, the success of RISUG® as an effective vas occlusion procedure has been well-established in different animal models and non-human primates.[15] The present investigation represents the first comprehensive long-term observations of semen analysis at functional, hormonal, biochemical, and ultrastructural levels in human subjects from the Phase III clinical trial on RISUG®.
The foremost parameter for analyzing an efficient male contraceptive is a demonstration of azoospermia (absence of sperm in the ejaculate) and the early onset of azoospermia is one of the key regulators in determining the efficacy of a contraceptive procedure. In vasectomy sterility is not obtained immediately after the surgical procedure, in most cases, it takes 8–12 weeks after vasectomy.[30] Similarly, hormonal contraceptives demonstrate a mean time of 10–15 weeks before the onset of oligospermia and/or azoospermia.[31] The previous reports in rats show 100% sterility within 15 days of RISUG® injection evident by various morphological aberrations in sperms.[32] Our results demonstrate that just after 3 weeks of RISUG® injection majority of subjects, that is more than 75% of the subjects show severe oligospermia and 25% of subjects showed azoospermia. Further 6 weeks post-injection, 98% of subjects were found to be azoospermic. Following RISUG blockage, spermatozoa remained in the epididymis and the sperm disposal mechanism initiates. This indicates the high contraceptive efficacy of RISUG® with the early onset of azoospermia in contrast to other male contraceptive methods.
Basic semen analysis has limited predictive value for fertilization, the sequential analysis of sperm function after contraceptive usage can better define the efficacy and effectiveness of a contraceptive in preventing pregnancy. A few studies comment on the functional competence of the spermatozoa in reference to sperm motility and vitality after vasectomy; however, no detailed study has been reported as yet.[8,33] Sperm functional assays performed in the present study define fertilizing capacity of residual spermatozoa from men rendered oligozoospermic by RISUG® injections that accentuate early contraception. In all the functional parameters studied, a decrease was observed after RISUG® injection implying the inability of spermatozoa in binding to ZP and causing capacitation. Lower values of HOS indicated a decrease in integrity of sperm plasma membrane which is an important factor in successful fertilization.[34] Similarly, values of all the sperm functional parameters observed in the sub-fertile or infertile range indicating diminished competency of spermatozoa in terms of cellular maturity, viability, acrosome intactness, etc. implies that residual sperms after RISUG® injection to be functionally inactive. Similar observations were made using ultrastructure studies where in a significant increase in sperm abnormalities was revealed in the form of head-tail separation, damaged, bulging and bent mid-piece, coiling of tail, etc. This further implies the advantage of RISUG® over other contraceptives as our results clearly demonstrate early azoospermia along with functional inactivation of voided spermatozoa.
The human seminal plasma is a complex mixture of the products of several glands consisting of various organic and inorganic components. Investigations on the levels of various biochemical constituents may provide important information about the contribution of different components of the reproductive system.[35] Studies after vasectomy imply blockage of vas deferens leading to atrophy of the prostate and a few studies report hyperfunction of the prostate after vasectomy indicated by increased levels of acid phosphatase in seminal plasma.[36,37] Similarly, fructose secreted by seminal vesicles is elevated in the semen of vasectomized men.[38] Semen analysis after RISUG® injection demonstrated no change in seminal vesicle secretion (fructose) and prostatic marker (acid phosphatase). In langur monkeys, no drastic changes in the secretory activity of the seminal vesicle and prostate have been reported.[39] Lack of alterations in the seminal vesicle and/ or prostatic secretions are important to ensure the successful reversibility of a contraceptive.[40] In human subjects under study, neutral α-glucosidase, considered a marker of epididymal function,[41] was found to decrease significantly after RISUG® injection as also observed in the case of GPC, another epididymal marker. Lowered levels of seminal GPC are observed in men with agenesis of the vas deferens or following vasectomy.[42] However, seminal plasma GPC levels have been reported to be restored to normal following vas reanastomosis.[37] Epididymis in mammals, including that of human, plays an important role in the development of a fertile ejaculate.[43] The intactness of vas deferens and epididymis is important for the maturation of spermatozoa and post-vasovasostomy alterations in vas deferens and epididymis lead to failure of functional reanastomosis.[13,44] The present data imply that RISUG® impacts epididymal function with no impairment of other accessory sex organs function thus ensuring a better potential for regain of fertility.
Hormones such as testosterone, FSH, and LH play a regulating role in the reproductive function of male.[45] In cases of azoospermia where spermatogenesis is arrested before spermatid differentiation, levels of FSH are reported to be elevated. Similarly, surgical stress has been noted to result in a decrease in plasma testosterone and LH levels.[46,47] Short-term studies after NSV have reported a lack of appreciable changes in the hormonal profile; however, long-term analysis on plasma hormone levels after vasectomy reports elevated LH and testosterone levels with no significant change in FSH levels.[48] After vasectomy backward pressure results in decreased testosterone levels that are compensated by increasing levels of LH. Stimulating factors may be absorbed in the plasma as the ductus stop producing a rise in LH normalizing serum testosterone levels.[48] Therefore, there are lesser chances of achieving fertility after vasectomy reversal. However, in azoospermic males after RISUG® injection, no change in any of the hormones was reported. This clearly implies that RISUG® blocks sperm passage without impacting hormonal function.
Prostate cancer is the second most common cancer detected in males worldwide, with a high mortality rate and PSA is the most frequently used marker for prostate cancer diagnosis.[49,50] There had been reports relating vasectomy to prostate cancer as detected by increased concentration of serum PSA following vasectomy procedures; however, conflicting data have also been reported.[51,52] Toward exploring the effects of RISUG® on prostate function, we determined circulatory levels of PSA and during the study period of 5 years, no significant changes were observed in comparison to pre-RISUG® injection.
Based on RISUG® methodology, VASALGELTM is being developed by the Parsemus Foundation, a non-governmental organization, since 2010.[53] The Parsemus Foundation has performed pre-clinical studies in rabbits[54] and monkeys[55] and intended to begin trials in humans in 2019. Similarly, another company in the USA, Contra line, is developing a hydrogel called Echo-V® an intravasal injection to block the flow of sperm.[14] Both VASALGEL™ and Echo-V® are far away from reaching markets as no human studies are done yet, no toxicological studies have been reported and it is not yet known how long both products will hold up once they enter the human body.[14]
After contraceptive efficacy, a major criterion of an ideal contractive is the possibility of reversal to reestablish fertility when required. Detailed short- and long-term studies on reversal of vas occlusion in male rats and rabbits using DMSO and NaHCO3 demonstrated effective recovery of fertility without any toxicity at the cellular levels.[24,56] In langur monkeys, sperm ultrastructure and sperm functional tests indicated the return of fertilizing ability of spermatozoa, hence, successful reversal of RISUG® injection using a non-invasive method.[39,57] On the contrary, vasectomy leads to different types of complications[58] including the development of anti-sperm antibodies in a significant proportion of men post-vasectomy. In studies as early as 1979, it was found that 60–80% of men showed the formation of spermatozoa agglutinating antibodies within 1 year of vasectomy and identified as a major reason for men not regaining fertility even after anatomically successful reanastomosis.[59,60] High levels of anti-sperm antibodies are also found in males with a clinical history of testicular torsion, varicocele, epididymis orchitis, seminal infection, etc.[61] However, in the present study, we observed a lack of alterations in ASA production after RISUG® injection. Vas occlusion in langur monkeys also indicated no change in levels of anti-sperm antibodies from their pre-treatment values until 540 days with successful repeated vas occlusion and non-invasive reversal procedures.[39,57] Pre-clinical reversal data and the absence of anti-sperm antibodies present a highly favorable condition for the future reversal procedure and fertility recovery in subjects after RISUG® reversal.
This is the first report, to the best of our knowledge, where in detailed functional attributes of residual spermatozoa has been reported after contraceptive usage to demonstrate the efficacy of the drug. Thus, the present study evidently demonstrates single intervention intravasal injection of RISUG® to be a safe and highly sustainable contraceptive with early contraceptive action and high possibility of functional reversal.
CONCLUSION
The functional reversal following male contraception has been a matter of concern for acceptability and participation of male partners in family planning program. In our study, long-term follow-up during Phase-III clinical trials, with focus on sperm functional parameters, demonstrates RISUG® as an early and efficient contraceptive. The lack of appreciable changes in hormone and selective biochemical parameters, known to interfere with functional reversibility following NSV, are suggestive of acceptable assurance of reversibility after intravasal RISUG® injection.
DECLARATIONS
Ethics approval and consent to participate
All research procedures were carried out following the guidelines of the Drug Controller General (India), Directorate General of Health Services, New Delhi, and were approved by the Ethics Committee, SMS Medical College and Hospital, Jaipur, vide letter dated May 4, 2002. Informed written consent was obtained from every subject recruited into this study.
Availability of data and materials
The datasets used and analyzed during the present study are available from the corresponding author upon reasonable request.
Authors’ contributions
All authors made substantial contributions to the conception and design, acquisition of data and analysis, and interpretation of data; SP, BK, and RKD carried out clinical investigations. TCS and ASA were involved in RISUG® injection procedures and follow-up. ASA, TCS, and PBK participated in drafting the article. NKL provided intellectual inputs and gave final approval for the version to be submitted. Each author participated sufficiently in this work and takes public responsibility for appropriate portions of the content.
Acknowledgments
The study was supported by the Indian Council of Medical Research, New Delhi. The infrastructural facilities provided by the Head of the Department are gratefully acknowledged. The authors are thankful to Prof. Sujoy K. Guha, Honorary Professor, Indian Institute of Technology (IIT)/All India Institute of Medical Sciences, New Delhi, for providing RISUG® and Dr. R. S. Sharma, Former Head, Division of Reproductive, Biology, Maternal and Child Health (RBMCH), Indian Council of Medical Research, New Delhi, for coordination of RISUG® Phase-III Clinical Trials. We thankfully acknowledge the support of the Ministry of Health and Family Welfare, Government of India and also the National Institute of Health and Family Welfare (NIHFW), New Delhi, for providing kits for sperm functional tests. The ultrastructural studies were carried out at the electron microscope facility of the University Science Instrumentation Centre (USIC), University of Rajasthan, Jaipur. The authors also thank all the subjects who participated in the RISUG® Phase-III Clinical Trials.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This work was supported by ICMR Project: Phase III clinical trial with an intravasal injectable male contraceptive - RISUG® Project No. 5/10/11/2006-RHN dated July 23, 2007, till July 31, 2021.
Conflicts of interest
There is no conflict of interest.
References
- Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann. 2014;45:301-14.
- [CrossRef] [PubMed] [Google Scholar]
- Unintended pregnancy and abortion: What does it tell us about reproductive health and autonomy? Lancet Glob Health. 2020;8:e1106-7.
- [CrossRef] [PubMed] [Google Scholar]
- Family Planning: A Global Handbook for Providers (3rd ed). Baltimore and Geneva: World Health Organization (WHO), Johns Hopkins Center for Communication Programs (CCP), U.S. Agency for International Development (USAID); 2018.
- [Google Scholar]
- Family planning, population growth, and the environment. Contraception. 2020;101:145-7.
- [CrossRef] [PubMed] [Google Scholar]
- Are men well served by family planning programs? Reprod Health. 2017;14:14.
- [CrossRef] [PubMed] [Google Scholar]
- National Family Health Survey (NFHS)-4. Ministry of Health and Family Welfare. Government of India 2015-2016. Available from: https://www.rchiips.org/nfhs/pdf/nfhs4/india.pdf [Last accessed on 2021 Aug 19]
- [Google Scholar]
- Contraceptive use and its effect on Indian women's empowerment: Evidence from the national family health survey-4. J Biosoc Sci. 2020;52:523-33.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and effectiveness of vasectomy. Fertil Steril. 2000;73:923-36.
- [CrossRef] [PubMed] [Google Scholar]
- Vasectomy in male contraception and its reversal. Eur Urol. 2014;13:68-72.
- [CrossRef] [Google Scholar]
- Male permanent contraception: Vasectomy In: Shoupe D, ed. The Handbook of Contraception. Switzerland, AG: Springer Nature; 2020. p. :255-72.
- [CrossRef] [Google Scholar]
- Review of vasectomy complications and safety concerns. World J Mens Health. 2020;38:e43.
- [Google Scholar]
- Barriers for low acceptance of no scalpel vasectomy among slum dwellers of Lucknow city. Indian J Public Health. 2019;63:10-4.
- [CrossRef] [PubMed] [Google Scholar]
- Vas deferens, a site of male contraception: An overview. Asian J Androl. 2001;3:87-96.
- [Google Scholar]
- Male contraceptive development: Update on novel hormonal and nonhormonal methods. Clin Chem. 2019;65:153-60.
- [CrossRef] [PubMed] [Google Scholar]
- RISUG: An intravasal injectable male contraceptive. Indian J Med Res. 2014;140:S63.
- [Google Scholar]
- RISUG as a male contraceptive: Journey from bench to bedside. Basic Clin Androl. 2020;30:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Safety & efficacy of an intravasal, one-time injectable and non-hormonal male contraceptive (RISUG): A clinical experience. Indian J Med Res. 2019;150:81-6.
- [CrossRef] [PubMed] [Google Scholar]
- WHO Laboratory Manual for the Examination of Human Semen and Sperm-cervical Mucus Interaction Cambridge: Cambridge University Press; 1999.
- [Google Scholar]
- Development of a rapid, sensitive, and reproducible laboratory test kit for the assessment of plasma membrane integrity of human sperm. Fertil Steril. 2008;89:223-7.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro decondensation of nuclear chromatin of human spermatozoa: Assessing fertilizing potential. Arch Androl. 1991;27:43-50.
- [CrossRef] [PubMed] [Google Scholar]
- Severe asthenozoospermia: A structural and functional study. Int J Androl. 1995;18:67-74.
- [CrossRef] [PubMed] [Google Scholar]
- Efficiency of routine semen analysis to predict functional and structural integrity of human spermatozoa. Indian J Exp Biol. 1995;33:652-4.
- [Google Scholar]
- Contraception with RISUG® and functional reversal through DMSO and NaHCO3 in male rabbits. Asian J Androl. 2017;19:389-95.
- [CrossRef] [PubMed] [Google Scholar]
- Atlas of Human Reproduction: By Scanning Electron Microscopy Germany: Springer Science & Business Media; 2012.
- [Google Scholar]
- Fructose, polyols, and organic acids In: Nam T, ed. The Biochemistry of Semen and of the Male Reproductive Tract. London: Methuen; 1964. p. :237-64.
- [Google Scholar]
- Acid phosphatase in seminal fluid-method of estimation and diagnostic significance. Andrologia. 1979;11:113-22.
- [CrossRef] [PubMed] [Google Scholar]
- Studies on the estimation of glycerol, fructose and lactic acid with particular reference to semen. Aust J Exp Biol Med Sci. 1959;37:441-50.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of tripchlorolide on the epididymides and testes of rats. Pituitary. 1999;28:25-79.
- [Google Scholar]
- Male hormonal contraception. Am J Obstet Gynecol. 2004;190:S60-8.
- [CrossRef] [PubMed] [Google Scholar]
- Sperm characteristics and teratology in rats following vas deferens occlusion with RISUG and its reversal. Int J Androl. 2010;33:e198-206.
- [CrossRef] [PubMed] [Google Scholar]
- The functional competence of human spermatozoa recovered after vasectomy. Reproduction. 1984;70:575-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sperm plasma membrane integrity in fertile and infertile men. Andrologia. 1992;24:141-4.
- [CrossRef] [PubMed] [Google Scholar]
- Male contraception In: Kumar A, Sharma M, eds. Basics of Human Andrology. Singapore: Springer; 2017. p. :493-508.
- [CrossRef] [Google Scholar]
- Endocrine and accessory sex organ function after vasectomy and vasovasostomy. Arch Androl. 1981;7:187-91.
- [CrossRef] [PubMed] [Google Scholar]
- Seminal fructose and acid phosphatase in vasectomised men. Int J Fertil. 1977;22:60-2.
- [Google Scholar]
- Repeated vas occlusion and non-invasive reversal with styrene maleic anhydride for male contraception in langur monkeys. Int J Androl. 2000;23:36-42.
- [CrossRef] [PubMed] [Google Scholar]
- Protein secretion and secretory processes in male accessory sex glands. Int Rev Cytol. 1990;121:127-231.
- [CrossRef] [PubMed] [Google Scholar]
- The relevance of neutral α-glucosidase activity in andrology. Syst Biol Reprod Med. 2009;55:116-9.
- [CrossRef] [PubMed] [Google Scholar]
- Glycerophosphocholine in seminal plasma of fertile and infertile men. Int J Androl. 1988;11:405-13.
- [CrossRef] [PubMed] [Google Scholar]
- On the epididymis and its role in the development of the fertile ejaculate. J Androl. 1995;16:292-8.
- [Google Scholar]
- Ultrastructure of langur monkey epididymidis prior to and following vasectomy and vasovasostomy. Eur J Morphol. 2000;38:24-33.
- [CrossRef] [PubMed] [Google Scholar]
- Male reproductive hormones and semen quality. Asian Pacific J Reprod. 2019;8:189.
- [CrossRef] [Google Scholar]
- Plasma testosterone levels following surgical stress in male patients. Eur J Endocrinol. 1970;65:11-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of surgical stress on pituitary-testicular function. Clin Endocrinol. 1978;9:255-66.
- [CrossRef] [PubMed] [Google Scholar]
- An investigation of plasma hormone levels before and after vasectomy. Fertil Steril. 1976;27:144-51.
- [CrossRef] [PubMed] [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- [CrossRef] [PubMed] [Google Scholar]
- PSA markers in prostate cancer detection. Urol Clin North Am. 2003;30:677-86.
- [CrossRef] [PubMed] [Google Scholar]
- Serum prostate-specific antigen concentration before and after vasectomy. Mil Med. 1996;161:356-7.
- [CrossRef] [PubMed] [Google Scholar]
- Vasectomy and risk of aggressive prostate cancer: A 24-year follow-up study. J Clin Oncol. 2014;32:3033.
- [CrossRef] [PubMed] [Google Scholar]
- Azoospermia in rabbits following an intravas injection of vasalgel. Basic Clin Androl. 2016;26:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Reversibility of vasalgel male contraceptive in a rabbit model. Basic Clin Androl. 2017;27:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- The contraceptive efficacy of intravas injection of Vasalgel™ for adult male rhesus monkeys. Basic Clin Androl. 2017;27:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Toxicity and mutagenicity evaluation following RISUG contraception reversal in rats. Int J Toxicol. 2018;37:457-65.
- [CrossRef] [PubMed] [Google Scholar]
- Preclinical evaluation for noninvasive reversal following long-term vas occlusion with styrene maleic anhydride in langur monkeys. Contraception. 2005;71:214-26.
- [CrossRef] [PubMed] [Google Scholar]
- Complications of vasectomy. Ann R Coll Surg Engl. 2005;87:406-10.
- [CrossRef] [PubMed] [Google Scholar]
- Immunological consequences of vasectomy In: Cunningham GR, Schill WB, Hafez ESE, eds. Regulation of Male Fertility. Clinics in Andrology. Vol 5. Dordrecht: Springer; 1980. p. :197-206.
- [CrossRef] [Google Scholar]
- Risk factors for antisperm antibodies in infertile men. Am J Reprod Immunol. 1994;31:69-76.
- [CrossRef] [PubMed] [Google Scholar]







