Translate this page into:
Prevalence of polycystic ovarian syndrome and its association with circulatory gonadotropins (luteinizing hormone and follicle-stimulating hormone) and prolactin in different reproductive age groups: A brief survey

*Corresponding author: Seema Rai, Department of Zoology, Guru Ghasidas Vishwavidyalaya, Bilaspur, Chhattisgarh, India. drseemakamlesh@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Purohit S, Rai S, Kalvit S. Prevalence of polycystic ovarian syndrome and its association with circulatory gonadotropins (luteinizing hormone and follicle-stimulating hormone) and prolactin in different reproductive age groups: A brief survey. J Reprod Healthc Med 2021;2:8.
Abstract
Polycystic ovarian syndrome can affect fertility due to anovulatory cycles, luteal phase defects, hyperprolactinemia, and sex hormone imbalance, it remains untreated. The present study aims prevalence of polycystic ovarian disease (PCOD) of clinical/subclinical infertile women, different age groups and to analyze the association between circulatory level of gonadotropins, luteinizing hormone and follicle-stimulating hormone (LH and FSH) and prolactin (PRL) in polycystic ovary syndrome (PCOS) women of different reproductive age and its impact on fertility of women. The hormonal reports for LH, FSH, and PRL of 100 female patients were analyzed. Women suffering from oligomenorrhea and amenorrhea are given priority in this study. These samples were categorized into five different age groups of 15–20 years, 21–25 years, 26–30 years, 31–35 years, and 36–40 years. Obtained hormonal data of LH, FSH, and PRL were pooled and the average was taken to compare with the normal range of hormone. A significant age-dependent variation observed in circulatory serum levels of gonadotropins (LH and FSH) and PRL. The study reveals that the highest PCOD patients were observed in the age group of 21–25 years. Whereas, 30% to 15–20 years, 60% to 21–25 years, 40% to 26–30 years, 30 % to 31–35 years, and 20 % to 36–40 years of reproductive age group. Most affected population of PCOS women ranges in between 21 and 25 age groups, whereas the 36–40 age group was least affected. Age-related alteration in the circulatory level of PRL (hyperprolactinemia) and pulsatility of LH and FSH can be considered as the important factor regulating neuronal mechanisms of hypophyseal gonadal and peripheral endocrine feedback.
Keywords
Polycystic ovary syndrome
Infertility
Luteinizing hormone
Follicle-stimulating hormone
Prolactin
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a common endocrine disorder of reproductive age affecting 2–8% of women worldwide.[1] It is a multifactorial, complex genetic disorder. The various characteristics of symptoms are chronic anovulation, oligomenorrhea, or acne.[2,3] It is associated with cardiovascular disorder, infertility, and psychological disorder. PCOS condition is represented by 10 tiny cysts of about 2–9 mm, which may occur in one or both the ovaries.[4] The hypothalamic-pituitary-ovarian (HPO) axis responds to internal signals (hormonal and neuronal) and external factors (environmental factors). Polycystic ovarian syndrome is a disorder primarily characterized by androgen excess (AE) and ovulatory dysfunction that disrupts the HPO axis function. Nonetheless, the characteristics neuroendocrine hallmark of PCOS is marked by an increase in serum concentration of luteinizing hormone (LH), also fluctuation in LH/follicle-stimulating hormone (FSH) ratio.[5] The average production of androgens and estrogen is increased in PCOS women as reflected by elevated serum concentration of testosterone, androstenedione, DHEA, and estrone. The persistence of androgen secretion gives negative feedback to estrogen, resulting in an increase of FSH secretion from the anterior pituitary. Follicular sensitivity of FSH increases in the ovary due to androgen accumulation.[5] Serum estrone concentrations are elevated due to peripheral conversion of an increased amount of androstenedione.[6,7] In contrast, serum estradiol levels in women with PCOS fluctuate but generally remain within the range observed in the follicular phase.[5,8] Current perspective views PCOS as a complex disorder, similar to cardiovascular disease (CVD) and Type-2 diabetes mellitus, wherein numerous genetic variants and environmental factors interact, combine, and contribute to the pathophysiology.[9] The evidence suggests that PCOS has been associated with increased oxidative stress secondary to mitochondrial function.[8] Oxidative stress can itself induce insulin resistance and hyperandrogenism in PCOS patients.[10] Childhood obesity is a well-documented risk in PCOS due to the development of insulin resistance, metabolic syndrome, and PCOS in the later part of life.[11] PCOS obese females increased visceral and subcutaneous body fat distribution due to increased androgen production.[12]
Apart from its role in the stimulation of milk production in nursing women, prolactin (PRL) participates in various physiologies throughout the body.[13-15] A report from the US National Library (2013) of Medicine suggests that hyperprolactinemia (elevated PRL level) could be a common problem in reproductive-aged women. Ongoing research finding very well establishes that in response to inflammation and hyperglycemia macrophages may secrete PRL hence promoting secretion of insulin from beta cells of islets of Langerhans.[16,17] PCOS women could be an important subclass with reduced infertility/infertile women and are associated with endocrine and gynecologic abnormalities that affect ovarian quality and function.[18,19] Both hyperprolactinemia and PCOS had endocrine disorders and irregular menstrual cycles. Nonetheless, PCOS is closely associated with increased inflammation, hypertension, increased incidence of CVD, hyperlipidemia, impaired glucose tolerance, insulin resistance, and metabolic syndrome.[20,21] Despite the various available research finding, there is still a lacuna toward the age-dependent correlation between circulatory PRL in PCOS patients; therefore, the present study was designed to assess the percentage of PCOS patients in different reproductive age groups as well as to analyze the circulatory hormonal level of gonadotropins (LH and FSH) and PRL.
MATERIAL AND METHODS
A survey was made in nearest hospital, Dr. Kavita Test Tube Baby Centre and Nursing Home, situated at, Seepat Chawk, Sarkanda, Bilaspur (C.G). The hormonal reports of 100 female patients were analyzed with the permission of the authority. These samples were categorized into five groups starting from (15 to 20 years, 21 to 25 years, 26 to 30 years, 31 to 35 years, and 36 to 40 years). The analyzed hormone was (LH, FSH and PRL) pooled and the average was taken to compare with the normal range of hormone. The main objective of this study to find the percentage of the polycystic ovarian syndrome in women with different reproductive age groups and to find the risk factor associated with the disorder. The main features that are taken into the study are missed periods, amenorrhea, oligomenorrhea, ovaries that are large or appearance of cysts, excess body hair, weight gain, infertility, and dark patches on the neck and back. Women suffering from oligomenorrhea and amenorrhea are given priority to this study. Amenorrhea was defined as the absence of a menstrual cycle in the last few months. Oligomenorrhea is defined as the menses >35 days. Infertility was diagnosed only in married women and was defined as the failure of pregnancy in the absence of male infertility and other factors. To gain status about the ovary, all the patients are subjected to ultrasonography for polycystic ovarian morphology and ovarian volume. Every patient questioned their menstrual status such as the age of menarche, about their menstrual cycle, and also about their habits and lifestyles.
Informed consent has been taken from infertile patients who visited the hospital that the data generated during treatment may be used for publication.
Statistical analysis
Data were analyzed using Student’s t-test followed by oneway ANOVA as appropriate. The difference was considered significant when P ≤ 0.05.
Diagnosis
Three distinct diagnostic criteria for PCOS have been fixed which are as follows:
Hyperandrogenism and irregular menstrual cycle as concluded by National Institute of Child Health and Human Development, 1990.
Oligo/anovulation, clinical, or/and/biochemical signs of hyperandrogenism/polycystic ovary laid down by European Society for Human Reproduction and Embryology and American Society for Reproductive Medicine, 2003.
PCOS Society (AE-PCOS) in 2006 concluded that diagnosis requires hyperandrogenism, ovarian dysfunction excess androgen level, and its associated disorder.
RESULTS
A significant age-dependent variation was observed in the circulatory serum level of gonadotropins (LH and FSH) and PRL. Maximum PCOD patients were observed in the age group of 21–25 years. Observation reveals that 30% of PCOD females were noted between 15 and 20 years of reproductive age group. About 60% of PCOS women were from 21 to 25 years of reproductive age group. 26–30 years of reproductive age group showed 40% PCOD women. 31–35 years and 36– 40 years of reproductive age group have 30% and 20% PCOD women, respectively [Figures 1 and 2].

- Variation in number of mild, moderate, and severe polycystic ovary syndrome patients in different reproductive age groups; n = 100 (20 in each age group) histogram represents mean ± SE; P < 0.001.

- Pie diagram representing variation in mild, moderate, and severe polycystic ovary syndrome patients (in percentage) of different reproductive age groups.
Results clearly showed that the most affected women population fall between 21 and 25 years of reproductive age group. The least affected population ranges between the age group of 36 and 40 years. USG criteria for the diagnosis of PCOS include the presence of 12 or more follicles in one ovary, each follicle measures 2–9 mm in diameter and ovarian volume >10 cm cube. Endocrine abnormalities include elevated LH and normal FSH. LH-FSH ratio became 3:1 [Figures 3 and 4].

- Circulatory levels of luteinizing hormone (left panel) and follicle-stimulating hormone (right panel) in normal control and polycystic ovary syndrome women of different reproductive age groups; n = 100 (20 in each age group) histogram represents mean ± SE; P < 0.05.

- Circulatory levels of prolactin; P < 0.05, in normal control and polycystic ovary syndrome women of different reproductive age groups n = 100 (20 in each age group) histogram represents mean ± SE; P<0.05.
The result indicated that most of the women became hyperprolactinemic. Increasing PRL levels cause ovulatory dysfunction, ranging from short luteal phase to anovulatory cycle to amenorrhea and hypogonadism, depending on the extent of gonadotropin secretion [Figure 4]. However,the associated phenotype approaches observed during PCOS showed highest percentage for hirsutism (40%), followed by acne (30%), while baldness and acanthosis were in lower range [Figure 5].
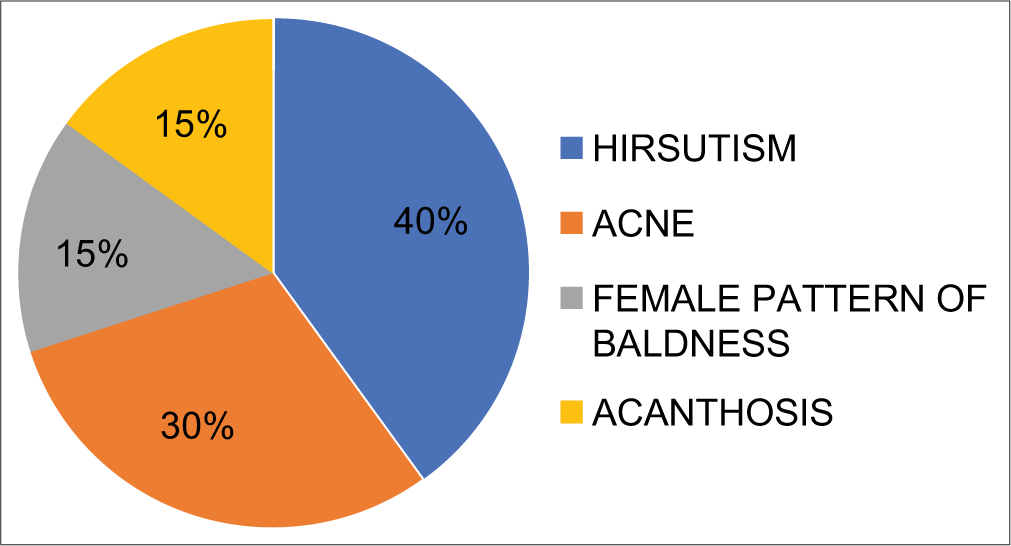
- Pie diagram showing an observational pattern of associated phenotype during PCOS.
DISCUSSION
Age is one of the factors influencing PRL secretion including many other factors. In the present observational study, we divided the PCOS patients into different reproductive age groups to exclude the influence of age. The results showed a general trend in the elevation of circulatory levels of PRL in PCOS patients when compared with control subjects. This coincides with previous findings.[22] An increase in the circulatory level of PRL during the normal course has an inhibitory effect on reproductive hormone because of that PCOD women are observed with hyperprolactinemia. A high incidence of obesity in a range of 35–40 % has been reported among the women suffering with chronic anovulation and PCOS. Obese women reported more susceptible for insulin resistance as compared to the lean women with PCOS, to an overall prevalence range of 50% and 75%. Insulin sensitivity decreases up to 35–40% in women with PCOS as compared to normal women. Up to 35% of women with PCOS exhibit impaired glucose tolerance and 7–10% meet criteria for Type-2 diabetes mellitus. The present survey reveals that there is an impaired secretion of circulatory gonadotropins (LH and FSH). LH/FSH increases in a 3:1 ratio. This situation of gonadotropin secretion might have affected the improper secretion of estrogen. Disrupted secretion of circulatory estrogen would have resulted in the impaired folliculogenesis lead to failure of ovulation resulting in PCOS conditions among women.
Further, the HPO axis is appearing to be very sensitive because of the impairment of the gonadotropin secretions. It appears that this improper pulsatility of LH and FSH might have stimulated the secretion of PRL. It may be assumed that such pathogenicity of PCOD may influence the anterior pituitary cells responsible for the secretion of PRL. The study showed that PCOD women have high gonadotropin-releasing hormone (GnRH) frequency because of disruption in GnRH pulsatile activity. A significant negative correlation was observed between circulatory serum LH/FSH and PRL levels of PCOS patients. This can be explained on the basis that high serum PRL above the normal range may suppress the synthesis of GnRH from the hypothalamus by inhibiting its synthesis under certain physiological and pathological conditions, which, in turn, reduces the secretion of GnRH to the portal vein.[23,24] Increase in circulatory serum PRL can also suppress kisspeptin neurons in the arcuate nucleus and hence can lead to suppressing the pulsatility of rhythmic frequency and amplitude of FSH and LH.[25-27] Reports provide strong link for influence of serum PRL in the regulation of pulsatile frequency and amplitude of gonadotropins, which, in turn, has direct involvement for serum estradiol and folliculogenesis. Failing to which might have led to anovulatory condition which may be considered responsible cause for PCOS infertile women.
Finding suggests a high level of LH in PCOD women would be responsible for the decrease of dopamine, due to this hyperprolactinemia occurs.[28] Nonetheless, increase in PRL level may range from short luteal phase to amenorrhea condition.[29] Another assumption is that during PCOD the condition of amenorrhea appeared, this mimics the pregnancy condition (pseudo) could be a strong reason for an increase in circulatory PRL level.[5] Hyperprolactinemia can be associated with high androgen levels because PRL receptors are present in the adrenal gland stimulate the release of DHEA.[30] Reports are suggesting increased circulatory insulin levels as the cause of hyperandrogenism in women with PCOS in two ways either by stimulating ovarian androgen production or by inhibiting hepatic SHBG production.[31]
In the present investigation, based on observations from 100 infertile women out of which 37 were noted as PCOS patients, suggest that serum PRL could be a useful marker in the diagnosis of PCOS. Further, our findings offer a very interesting observation regarding hyperprolactinemia in PCOS women of particularly the age range of 21–25 years, 31–35 years, and 36–40 years establishing a correlation between PRL and aging. The women of age between 21 and 25 years are noted the most susceptible for metabolic dysregulation which could be strong reason to predict for future risk of diabetes and CVDs on that leading to insulinemia and obesity as reported earlier[32,33]
Current literature supports the notion that the presence of the MetS in youth may be an important predictor of future risk for diabetes and CVD.
It is not only PRL but also age-associated alterations that were noted in serum LH and FSH as well as addressing the weakening of ovarian reserve in those patients. The prevalence of PCOS based on observation and analysis of the present data, it is very clear that the prevalence of the occurrence of PCOD in young age reproductive females could be because of the various kinds of stresses related to studies and career settlement. Further, the various kinds of stresses can be considered as one of the important factors affecting the hypophyseal pituitary-gonadal axis, therefore, altering the circulatory level of gonadotropin hormones (LH and FSH) as well as the increased circulatory level of PRL.[19,34] During survey as well as the earlier report suggest that PCOS patients tend to adopt low quality of life and low mood, which can be because of the elevation of dopamine secretion and alterations in serum PRL levels.[19,34,35] Development of impaired glucose tolerance and insulin resistance in patients of hyperprolactinemia were reported which might be a genuine reason for developing PCOS.[36] This relationship is reversed in the subjects having a normal physiological range of circulatory PRL.[20]
Data suggest a direct impact of age on PRL secretion. Findings suggest a significant negative correlation between serum PRL and gonadotropins (LH/FSH). The present studies are expected to be relevant because PRL can also be considered along with the androgen and gonadotropins (LH and FSH), as a very important and useful marker during the diagnosis of PCOS. After all, this endocrinopathy among reproductive age group women is nowadays the principal reason, leading to infertility because of anovulatory and/or oligoovulatory conditions. Such study needs more attention and exploration to gather additional statistics on PCOS to contribute a better understanding regarding the age-associated changes in circulatory PRL as well as LH and FSH in PCOS women before reaching any conclusion.
CONCLUSION
Present finding indicates that PCOS is not always associated with hyperprolactinemia. Nonetheless, hypo/ hyperprolactinemia and polycystic ovary syndrome (PCOS) are observed as the most common causes of female infertility. Further, based on medical literature both are clinically distinct. However, since their pathologies share various clinical features therefore PCOS infertile women must be accessed for serum prolactin as well as for the other clinical / pathological examination to confirm the actual cause of coexistence of these two disorders.
Acknowledgment
Authors thank Dr. Subhada Kalvit, Director, Kalvit Infertility Centre, Bilaspur, for extending the assistance to analyses the patient’s data for this study. Further, we duly take this opportunity to express our deep sense of gratitude to Professor Neeta Singh and Dr. Monika Saini for their valuable guidance, inspiration, and intellectual encouragement during the preparation of the manuscript.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Prevalence of the polycystic ovary syndrome in unselected black and white women of the Southeastern United States: A prospective study. J Clin Endocrinol Metabolism. 1998;83:3068-82.
- [CrossRef] [Google Scholar]
- The androgen excess and PCOS society criteria for the polycystic ovary syndrome: The complete task force report. Fertil Steril. 2009;91:456-88.
- [CrossRef] [PubMed] [Google Scholar]
- The polycystic ovary, I. Clinical and histologic features. J Clin Endocrinol Metab. 1962;22:325-38.
- [CrossRef] [PubMed] [Google Scholar]
- Poly cystic ovarian syndrome: An updated overview. Front Physiol. 2016;7:124.
- [CrossRef] [PubMed] [Google Scholar]
- The pathogenesis of polycystic ovary syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520.
- [CrossRef] [PubMed] [Google Scholar]
- Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13:946-57.
- [CrossRef] [PubMed] [Google Scholar]
- Early-to-mid gestation fetal testosterone increases right-hand 2D: 4D finger length ratio in polycystic ovary syndrome-like monkeys. PLoS One. 2012;7:e42372.
- [CrossRef] [PubMed] [Google Scholar]
- Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:336-30.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic basis of polycystic ovary syndrome (PCOS): Current perspectives. Appl Clin Genet. 2019;12:249-60.
- [CrossRef] [PubMed] [Google Scholar]
- Mitochondrial complex I impairment in leukocytes from polycystic ovary syndrome patients with insulin resistance. J Clin Endocrinol Metab. 2009;94:3505-12.
- [CrossRef] [PubMed] [Google Scholar]
- PCOS forum: Research in polycystic ovary syndrome today and tomorrow. Clin Endocrinol (Oxf). 2011;74:424-33.
- [CrossRef] [PubMed] [Google Scholar]
- Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab. 1990;70:473-9.
- [CrossRef] [PubMed] [Google Scholar]
- Prolactin: The new biology of an old hormone. Annu Rev Physiol. 2002;64:47-67.
- [CrossRef] [PubMed] [Google Scholar]
- Prolactin regulates pain responses via a female-selective nociceptor specific mechanism. iScience. 2019;20:449-65.
- [CrossRef] [PubMed] [Google Scholar]
- Human cytomegalovirus infection induces high expression of prolactin and prolactin receptors in ovarian cancer. Biology (Basel). 2020;9:44.
- [CrossRef] [PubMed] [Google Scholar]
- Adipose tissue macrophages (ATM) of obese patients are releasing increased levels of prolactin during an inflammatory challenge: A role for prolactin in diabesity? Biochim Biophys Acta. 2014;1842:584-93.
- [CrossRef] [PubMed] [Google Scholar]
- Prolactin: A pleiotropic neuroendocrine hormone. J Neuroendocrinol. 2008;20:752-63.
- [CrossRef] [PubMed] [Google Scholar]
- Polycystic ovarian syndrome-prognosis and outcomes. Best Pract Res Clin Obstet Gynaecol. 2006;20:751-78.
- [CrossRef] [PubMed] [Google Scholar]
- The association between prolactin and metabolic parameters in PCOS women: A retrospective analysis. Front Endocrinol (Lausanne). 2020;11:263.
- [CrossRef] [PubMed] [Google Scholar]
- An update on the pathogenesis, inflammation, and metabolism in hirsutism and polycystic ovary syndrome. Gynecol Endocrinol. 2010;26:281-96.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetes and cardiovascular events in women with polycystic ovary syndrome: A 20-year retrospective cohort study. Clin Endocrinol (Oxf). 2013;78:926-34.
- [CrossRef] [PubMed] [Google Scholar]
- Prolactin secretion in polycystic ovary syndrome (PCOS) Neuro Endocrinol Lett. 2015;36:53-8.
- [Google Scholar]
- The 24 h pattern of pulsatile luteinizing hormone, follicle stimulating hormone and prolactin release during the first 8 weeks of lactational amenorrhoea in breastfeeding women. Hum Reprod. 1992;7:951-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pituitary portal plasma levels of oxytocin during the estrous cycle, lactation, and hyperprolactinemia. Ann N Y Acad Sci. 1992;652:397-410.
- [CrossRef] [PubMed] [Google Scholar]
- Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology. 2014;155:1010-20.
- [CrossRef] [PubMed] [Google Scholar]
- Prolactin-and testosterone-induced inhibition of LH secretion after orchidectomy: Role of preoptic and tuberoinfundibular gamma-aminobutyric acidergic neurones. J Endocrinol. 1994;143:165-74.
- [CrossRef] [PubMed] [Google Scholar]
- Estradiol potentiates but is not essential for prolactin-induced suppression of luteinizing hormone pulses in female rats. Endocrinology. 2020;161:bqaa022.
- [CrossRef] [PubMed] [Google Scholar]
- Increased luteinizing hormone sensitivity to dopamine inhibition in polycystic ovary syndrome. J Clin Endocrinol Metab. 1981;52:231-4.
- [CrossRef] [PubMed] [Google Scholar]
- Galactorrhea and hyperprolactinemia during treatment of polycystic ovary syndrome. Obstet Gynecol. 1980;55:460-3.
- [Google Scholar]
- Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol. 2010;22:42-52.
- [CrossRef] [PubMed] [Google Scholar]
- The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059-61.
- [CrossRef] [Google Scholar]
- The influences of hyperprolactinemia and obesity on cardiovascular risk markers: Effects of cabergoline therapy. Clin Endocrinol (Oxf). 2006;64:366-70.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur J Endocrinol. 2005;153:853-60.
- [CrossRef] [PubMed] [Google Scholar]
- Prescription of antidepressants is increased in Danish patients with polycystic ovary syndrome and is associated with hyperandrogenism. A population-based cohort study. Clin Endocrinol (Oxf). 2014;80:884-9.
- [CrossRef] [PubMed] [Google Scholar]
- Glucose abnormalities associated to prolactin secreting pituitary adenomas. Front Endocrinol (Lausanne). 2019;10:327.
- [CrossRef] [PubMed] [Google Scholar]






