Translate this page into:
Ionizing radiation and reproductive health: Impacts and mitigation strategies

*Corresponding author: Arun Chougule, Dean and Chief Academic Officer, Swasthya Kalyan Group, Sitapura, Jaipur, Rajasthan, India. arunchougule11@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chougule A, Joan M. Ionizing radiation and reproductive health: Impacts and mitigation strategies. J Reprod Healthc Med. 2025;6:6. doi: 10.25259/JRHM_33_2024
Abstract
Objectives
The objective of this study is to evaluate the impact of medical applications of ionizing radiation on reproductive health and to highlight strategies for minimizing associated risks while maintaining the therapeutic efficacy of medical interventions.
Material and Methods
A comprehensive review of the literature was undertaken to assess the reproductive risks associated with ionizing radiation from medical applications. Multiple databases were searched using predefined keywords: “Radiation therapy,” “Fertility preservation,” “Dose-dependent effects,” “As low as reasonably achievable (ALARA),” and “Mitigation strategies.” Manual searches of reference lists were also done using the same keywords. The review focused on diagnostic imaging modalities, including computed tomography scans and therapeutic procedures such as radiation therapy for malignancies. Particular attention was given to high-dose exposures and their potential to induce deoxyribonucleic acid (DNA) damage, gametogenesis disruption, hormonal imbalances, radiation-induced secondary infertility, and adverse pregnancy outcomes. Studies investigating dose-dependent effects on fertility, embryonic development, and congenital abnormalities were included in the study. In addition, mitigation strategies were evaluated, emphasizing the application of ALARA principles, advances in radiation shielding techniques, and the adoption of low-dose imaging technologies. Secondary factors, such as the role of patient education, reproductive counseling, and emerging protective agents, were also reviewed to provide a holistic understanding of risk management.
Results
The findings reveal that ionizing radiation from medical applications can pose substantial risks to reproductive health, particularly when exposure is repeated or involves high doses. DNA damage is a primary concern, which can lead to mutations that affect fertility and embryonic development. Radiation-induced disruptions in gametogenesis and hormonal imbalances further exacerbate reproductive challenges. Pregnant individuals and patients undergoing fertility-preserving treatments represent particularly vulnerable populations, given the heightened sensitivity of reproductive tissues and the potential for adverse pregnancy outcomes, including miscarriage and congenital abnormalities. However, mitigation strategies have shown promise in reducing these risks. Advances in low-dose imaging technologies have made it possible to achieve diagnostic accuracy with significantly reduced radiation exposure. Improved radiation shielding techniques, including lead aprons and pelvic shields, provide additional layers of protection, especially during procedures involving high radiation doses. Adherence to ALARA principles remains a cornerstone of safety, ensuring that radiation exposure is minimized without compromising diagnostic or therapeutic objectives. Furthermore, patient education and reproductive counseling play critical roles in promoting informed decision-making and awareness of potential risks. Emerging protective agents, such as radioprotective drugs, offer additional safeguards by mitigating radiation-induced cellular damage, although their widespread application requires further clinical validation.
Conclusion
While ionizing radiation is an indispensable tool in modern medicine, its potential to impact reproductive health necessitates careful and proactive management. A multifaceted approach is essential, combining technological advancements, rigorous adherence to safety protocols, and patient-centered strategies to optimize the benefits of medical radiation while minimizing associated risks. Healthcare professionals must prioritize education and counseling for vulnerable populations, ensuring that patients are informed about risks and available protective measures. Policymakers and researchers are encouraged to support the development and implementation of innovative mitigation strategies, such as advanced shielding technologies and radioprotective agents. By balancing therapeutic efficacy with the need to safeguard reproductive health, this approach provides a roadmap for improving patient outcomes and promoting long-term well-being in the context of medical radiation exposure.
Keywords
As low as reasonably achievable (ALARA)
Dose-dependent effects
Fertility preservation
Mitigation strategies
Radiation therapy
INTRODUCTION
Ionizing radiation is an indispensable tool in modern medicine, widely used in diagnostic imaging and therapeutic interventions for various diseases, including cancer. However, it is potential to cause cellular and molecular damage raises concerns, particularly regarding reproductive health.[1,2] The increasing cure rate of cancer has led to a vast population of survivors facing the late adverse effects of radiation treatments, with fertility impairment being one of the most sensitive issues for patients.[3,4] The sensitivity of the reproductive system to ionizing radiation stems from its reliance on rapidly dividing cells, such as germs cells and the intricate hormonal balance which is essential for fertility and pregnancy.[5,6] As the use of advanced radiological technologies grows, understanding and mitigating the reproductive risks associated with medical radiation becomes paramount.[7]
In the realm of cancer treatment, radiotherapy has evolved significantly, with a heightened emphasis on precision and organ preservation. Techniques such as intensity-modulated radiotherapy (IMRT), volumetric-modulated radiotherapy (VMAT), proton therapy, and stereotactic body radiotherapy have revolutionized the field by allowing high-dose radiation to target tumors while minimizing exposure to surrounding healthy tissues, including reproductive organs.[8,9] Concurrently, fertility preservation strategies, including ovarian transposition, cryopreservation of gametes, and the use of gonadotropin-releasing hormone (GnRH) agonists during treatment, have gained prominence. These advances reflect a growing commitment to maintaining the quality of life and reproductive potential of patients, especially among younger cancer survivors.[10,11]
This article explores the dual challenge of leveraging ionizing radiation for medical purposes while safeguarding reproductive health. It examines the biological effects of radiation on fertility and pregnancy, evaluates the risks associated with diagnostic and therapeutic procedures, and highlights recent innovations in radiotherapy aimed at preserving organ function and fertility. In addition, it addresses oncofertility strategies such as ovarian tissue cryopreservation and hormonal treatments, which provide options for patients undergoing high-risk cancer therapies.[12,13] By integrating these aspects, the article aims to provide a comprehensive framework for balancing therapeutic efficacy with the long-term reproductive well-being of patients.[14,15]
MATERIAL AND METHODS
A comprehensive search of multiple databases using predefined keywords: “Radiation therapy,” “Fertility preservation,” “Dose-dependent effects,” “ALARA,” and “Mitigation strategies” were done, and a total of 215 articles were identified through database searches, and an additional 12 articles were identified through manual searches of reference lists, resulting in a total of 227 articles for initial consideration.
Irrelevant articles based on the title or abstract were excluded from the study. Specifically, articles focusing on non-human studies, non-reproductive health topics, and non-ionizing radiation were removed. After this screening process, 102 articles remained for further assessment.
The 102 articles were reviewed to assess their relevance and methodological quality. Inclusion criteria were established as studies focusing on the impact of ionizing radiation on reproductive health and addressing fertility preservation, dose-dependent effects, or mitigation strategies published in peer-reviewed journals. Both quantitative and qualitative studies were included if they provided insights into reproductive health and radiation.
Studies not written in English, conference abstracts, editorials, and non-peer-reviewed articles, and studies with insufficient methodological detail or unclear outcomes were excluded from the study. After applying these criteria, 51 were included in the final review.
BIOLOGICAL EFFECTS OF IONIZING RADIATION ON REPRODUCTIVE HEALTH
Ionizing radiation interacts with biological tissues by depositing energy that leads to molecular damage, particularly to DNA. The reproductive system, which comprises rapidly dividing cells and hormonally sensitive organs, is especially vulnerable.[6,7] The effects on reproductive health depend on several factors, including radiation dose, exposure frequency, and the developmental stage at the time of exposure.[8,16]
EFFECTS ON MALE REPRODUCTIVE HEALTH
The male reproductive system comprises the testes, epididymides, vas deferens, seminal vesicles, and accessory glands. The testes are particularly sensitive to ionizing radiation due to their high proportion of proliferating germ cells.[4]
Spermatogenesis
Spermatogenesis is the process of sperm cell development, which can be adversely affected by exposure to radiation. Spermatogenic cells are particularly sensitive to radiation, and exposure can lead to varying degrees of infertility, depending on the dose received. Radiation doses above 1 Gy are known to significantly reduce sperm count, potentially resulting in temporary or permanent infertility. Lower doses may cause temporary disruptions in sperm production, while higher doses can result in long-term or irreversible damage. The extent of the impact on spermatogenesis depends on factors such as the dose, duration of exposure, and individual sensitivity to radiation.[17-23]
Hormonal changes
Damage to Leydig cells at higher doses (>20 Gy) may impair testosterone production.[1] Hormonal changes can occur due to radiation exposure, particularly when Leydig cells are damaged. Leydig cells, located in the testes, are responsible for producing testosterone, a key hormone in male reproductive function. When exposed to higher doses of radiation, typically >20 Gy, these cells may sustain damage, leading to impaired testosterone production. Reduced testosterone levels can have significant effects on male reproductive health, including decreased libido, erectile dysfunction, and compromised spermatogenesis. The severity of these hormonal changes depends on the radiation dose and the extent of damage to the Leydig cells.[17-24]
Genetic risks
Radiation-induced DNA damage in sperm may result in genetic mutations that can be passed on to offspring. Radiation exposure poses significant genetic risks due to its potential to induce DNA damage in sperm cells. This damage can result in genetic mutations, which may be passed on to offspring. These mutations can lead to a range of hereditary disorders or developmental issues in the next generation. The risk of such genetic damage increases with higher radiation doses and prolonged exposure. Ensuring proper radiation protection is crucial to minimize these risks and safeguard the genetic integrity of sperm, thereby protecting future generations from potential genetic disorders.[13,17-24]
EFFECTS ON FEMALE REPRODUCTIVE HEALTH
The female reproductive system includes the ovaries, fallopian tubes, uterus, and vagina. The ovaries, housing finite oocyte reserves, are highly sensitive to radiation damage.[12,25]
Oogenesis
Oogenesis, the intricate process of female germ cell development, is particularly vulnerable to radiation exposure. Even relatively moderate radiation doses between 2 and 3 Gy can cause substantial harm to these cells. Such exposure may lead to premature ovarian failure, a condition where the ovaries lose their normal function prematurely. This significantly affects fertility by reducing the ovarian reserve and impairing the body’s ability to produce viable eggs, ultimately impacting reproductive health.[12,25,26]
Uterine damage
Uterine damage from high radiation doses can significantly impair its function, potentially disrupting embryo implantation and hindering fetal development. This damage may lead to complications in pregnancy, including reduced fertility, increased risk of miscarriage, and potential long-term effects on the reproductive health of the uterus.[6,11,12,25-27]
Increased miscarriage risk
Prenatal radiation exposure significantly increases the risk of miscarriage, stillbirths, and congenital anomalies. Even low levels of exposure during critical stages of pregnancy can have profound impacts on fetal development, potentially leading to severe health complications or loss of the pregnancy. This underscores the importance of strict radiation safety protocols and careful monitoring to minimize exposure risks and protect both the mother and the developing fetus during pregnancy.[8,11,12,25-27]
The severity of radiation-induced damage is dose-dependent. Low-dose radiation, typically associated with diagnostic imaging, may cause subclinical effects, such as minor DNA damage that the body can repair. However, repeated exposures can accumulate and potentially affect gametogenesis over time.[1] High-dose radiation, common in therapeutic applications, can cause severe cellular and tissue damage, including destruction of germ cells and disruption of the hormonal milieu, leading to infertility or early menopause in women and azoospermia in men.[27] Threshold doses for reproductive damage have been identified, with gonadal tissue showing high sensitivity. For instance, a dose of 2–3 Gy can cause permanent infertility in women, while a dose of 0.1–1 Gy can transiently or permanently impair spermatogenesis in men.[8,17-24]
The reproductive system’s sensitivity to ionizing radiation varies across different life stages. In utero, developing embryos and fetuses are highly sensitive to radiation, with risks including miscarriage, congenital malformations, growth retardation, and neurodevelopmental deficits. The timing of exposure during pregnancy is critical:[7,11-14,25-27]
Pre-implantation stage (0–2 weeks)
Exposure may result in “all-or-nothing” effects – either the embryo survives without harm or fails to develop.
Organogenesis stage (2–8 weeks)
Radiation can cause severe malformations in the developing organs, including the reproductive system.
Fetal stage (>8 weeks)
The risks shift to growth retardation and functional impairments, as organ structures are mostly formed.
CHILDHOOD AND ADOLESCENCE
During these stages, gonads are actively developing, making them particularly susceptible to radiation-induced damage. In females, oocytes are highly radiosensitive, while in males, spermatogonia stem cells can be affected, potentially leading to impaired fertility in adulthood.[6,25] Protective measures during pregnancy shall include pregnancy testing before radiological or nuclear procedures in women of childbearing age, postponing non-urgent procedures until after pregnancy, and use of the “As Low As Reasonably Achievable” (ALARA) principle for diagnostic and therapeutic interventions.[27] Genetic counseling should be offered to individuals exposed to significant radiation doses who are planning conception.[14] In addition, long-term follow-up studies should be conducted to monitor potential trans-generational effects of radiation exposure.[7] These approaches are essential to understand the reproductive risks better and to provide informed guidance to those affected by radiation exposure.
Fertility challenges in men with cancer are less complex than those in women due to the relative ease of sperm banking. However, several factors can negatively affect male fertility, including disruptions of the hypothalamic-pituitary-gonadal (HPG) axis, damage to the germinal epithelium, and depression related to the diagnosis of cancer.[28] Different options to preserve the fertility of adult patients are routinely used in clinical practice. However, fertility preservation strategies for prepubertal patients at risk of infertility are limited to the cryopreservation of immature gonadal tissue. In recent decades, many research efforts have been focused on the future use of cryopreserved gonadal tissue. In premature ovarian insufficiency (POI), the threshold radiation doses decrease with increasing age of the patient, corresponding to 20.3 Gy in infants, 18.4 Gy in children up to 10 years, 16.5 Gy in adolescents up to 20 years, and 14.3 Gy in older women.[5,29]
In male, Leydig cell damage is induced by lower irradiation doses in prepubertal patients compared to adult patients.[17-24,30] Testicular irradiation at doses of 2–4 Gy has been shown to cause transient impairment of spermatogenesis. This disruption can lead to a period of azoospermia, where no sperm is present in the semen. However, recovery of spermatogenesis is possible after this temporary infertility, depending on the extent of damage. As long as the spermatogonial cells, which are the precursor cells for sperm production, are not completely depleted, recovery can occur over a variable period. The potential for recovery varies among individuals, with factors such as the exact dose and duration of exposure influencing the outcome.
Adulthood
Adult reproductive organs are somewhat less sensitive compared to developmental stages, but high doses of radiation can still lead to acute and long-term infertility.[17-20]
Other factors, such as genetic predisposition, overall health, and concurrent medical treatments (e.g., chemotherapy), also play a significant role in determining the extent of radiation-induced reproductive damage. Variations in DNA repair mechanisms and hormonal responses can influence individual resilience or susceptibility to radiation exposure.[8-10,17-24,31]
Understanding these factors is crucial for tailoring medical interventions, optimizing radiological safety, and developing strategies to protect reproductive health during and after radiation exposure. At the time of diagnosis of cancer, a significant proportion of post-pubertal patients have not completed their family planning and express a desire for pregnancy after treatment. However, despite all possible available fertility preservation options for males and females, cancer survivors have significantly reduced chances of post-treatment pregnancies compared with the general population.[25-27,32-34]
EFFECTS ON FEMALE REPRODUCTIVE HEALTH
Women are born with a finite number of oocytes, also known as ovarian reserve. This pool of oocytes declines over time until menopause or ovarian failure. As ovarian reserve declines, so does fertility. Furthermore, ionizing radiation poses significant risks to female reproductive health due to the high radiosensitivity of ovarian follicles and the intricate hormonal networks that regulate fertility. The severity of these effects depends on factors such as the dose, duration, and timing of exposure, as well as the woman’s age and overall reproductive health.[11-14,25,27,33]
Damage to ovarian follicles
The ovaries contain a finite number of primordial follicles, which represent a woman’s ovarian reserve. Ionizing radiation can cause direct DNA damage, inducing double-strand breaks in DNA that lead to apoptosis (programmed cell death) in oocytes.[6,7] Reduction in ovarian reserve occurs as the depletion of ovarian follicles reduces the reproductive lifespan and increases the risk of POI. Women exposed to radiation, especially during childhood or adolescence, may experience early menopause due to accelerated follicular depletion.[11-13,27]
Threshold radiation doses for ovarian damage indicate that 0.1 Gy can impair ovarian function, while doses over 2 Gy may cause permanent sterility, especially in premenopausal women. This underscores the importance of monitoring and minimizing radiation exposure to protect reproductive health.[13,26,35]
Disruption of the hypothalamic-pituitary-ovarian (HPO) axis
Ionizing radiation can disrupt the HPO axis, a critical regulator of female reproductive function. Radiation-induced damage to the hypothalamus or pituitary gland can impair the secretion of gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH), leading to anovulation and irregular menstrual cycles.[11,12,25,27] The diminished hormonal feedback loop between the ovaries and the brain can further compromise fertility and overall endocrine health.
Effects on pregnancy
Pregnant women exposed to ionizing radiation face unique risks to both maternal and fetal health. The impact depends on the timing and dose of exposure during pregnancy. Radiation exposure at the pre-implantation stage (0–2 weeks) may result in “all-or-nothing” effects; either the embryo is unaffected or fails to implant, leading to miscarriage.[8,16,28,29] 2–8 weeks is a critical phase when organogenesis or organ development takes place; radiation can cause structural malformations in the fetus, including reproductive organ abnormalities.[5,7,34] Exposure later in pregnancy (>8 weeks, called fetal stage) is less likely to cause malformations but can lead to growth restriction, neurodevelopmental delays, and functional impairments, including reduced fertility in offspring.[6,11-16]
Risk of congenital anomalies
Maternal exposure to ionizing radiation during pregnancy may increase the likelihood of congenital anomalies in the child, particularly when doses exceed 0.1 Gy. Such anomalies include genitourinary malformations if the radiation affects the developing reproductive organs, growth retardation and low birth weight, and neurological impairments or intellectual disabilities, depending on the fetal stage of exposure.[4,8]
Secondary risks in medical contexts
Radiotherapy or diagnostic imaging procedures involving the pelvic or abdominal region can inadvertently expose the ovaries and uterus to ionizing radiation. Cancer patients undergoing pelvic radiation therapy are at high risk of infertility and pregnancy complications due to the cumulative effects on ovarian and uterine health.[4,25] While modern imaging techniques use lower doses, repeated scans can pose cumulative risks, particularly for patients undergoing frequent monitoring.[6]
MITIGATION STRATEGIES FOR WOMEN
To minimize radiation-induced harm to female reproductive health, several strategies could be adopted.
Ovarian shielding
Ovarian shielding involves placing a protective barrier, usually made of lead or other radiation-attenuating materials, over the pelvic region to reduce exposure to ionizing radiation during diagnostic or therapeutic procedures. This is particularly important for young women and girls, as their ovarian reserve is highly sensitive to radiation. Shields are often used during diagnostic examinations such as X-rays, computed tomography (CT) scans, and fluoroscopy procedures involving the abdomen or pelvis to block scatter radiation from reaching the ovaries. In radiotherapy, shielding is applied when treating cancers near the pelvic region, such as colorectal or bladder cancer, to protect ovarian tissue.[12,13]
Shielding may not be feasible during certain treatments, such as pelvic radiotherapy for gynecological cancers, where precise targeting of the tumor may conflict with shielding efforts. Misplacement of shields can interfere with image quality or treatment precision, requiring careful planning by medical physicists. The development of customizable shields tailored to patient anatomy and the use of automated radiation monitoring systems to ensure shielding effectiveness during procedures helps to curtail these disadvantages. Gonadal shielding during pelvic radiotherapy by lead blocks can reduce the expected radiation dose to 4-5 Gy; however, the minimum free margin should be 2 cm to reduce the risk of gonadal irradiation due to inner organ movement.[5,30,32-35]
Ovarian transposition (oophoropexy)
Ovarian transposition outside the planned radiotherapy (RT) field is a routinely used technique to minimize ovarian follicle radiotherapy exposure. Ovarian transposition is a surgical procedure in which the ovaries are repositioned outside the anticipated radiation field, usually to a location higher in the abdomen. This method is aimed at preserving ovarian function and fertility in women undergoing pelvic radiotherapy. The surgery is typically performed laparoscopically, making it minimally invasive. The ovaries are detached from their original position and secured to the abdominal wall or other safe locations, depending on the radiation field’s configuration. It is often performed alongside tumor resection or other cancer treatments. Most applications are when pelvic cancers such as cervical, rectal, or bladder cancers where radiotherapy is required and when young women and adolescent girls wish to retain fertility after cancer treatment. The rate of retained ovarian function is approximately 65% in patients undergoing surgery and radiotherapy, and reasons for failure include necrosis related to vascular impairment and migration after insufficient fixation.[5,11,12,25,27,30,32-36]
Studies show that ovarian transposition can significantly reduce the risk of radiation-induced ovarian damage, but the procedure is not without risks, including ischemia (reduced blood flow) to the ovaries and the potential for ovarian cyst formation. Hormonal function may still be compromised if scattered radiation reaches the ovaries. If cancer metastasizes to the ovaries, this procedure is contraindicated. Optimization of the procedure could be achieved by the use of pre-operative imaging to optimize transposition planning and integration with other fertility-preserving techniques, such as ovarian tissue cryopreservation.[5,33-37]
Age, a major determinant for ovarian transposition and gonadal shielding, is indicated in women of <40 years with all other parameters of fertility are normal who will get treatment by radiotherapy for cervical, vaginal, rectal, anal cancers, Hodgkin’s or non-Hodgkin’s lymphoma in the pelvis or Ewing’s sarcoma of the pelvis with any metastasis anywhere in the body.
FERTILITY PRESERVATION TECHNIQUES
Cryopreservation of oocytes
Mature eggs are harvested from the ovaries, frozen, and stored for future use. Hormonal stimulation is used to induce the ovaries to produce multiple eggs. The eggs are retrieved through a minor surgical procedure, usually ultrasound-guided aspiration. Retrieved eggs are cryopreserved using vitrification, a fast-freezing technique that prevents ice crystal formation. This method is most suitable for post-pubertal females who have time for ovarian stimulation before starting radiation or chemotherapy.[12,26]
Cryopreservation of embryos
Fertilized embryos (created using in vitro fertilization [IVF]) are frozen for future implantation. This method is similar to oocyte cryopreservation but involves fertilization of retrieved eggs with sperm before freezing. It is ideal for women with a male partner or access to donor sperm.[4,5,30,32,33,35]
Cryopreservation of ovarian tissue
A portion of ovarian tissue is surgically removed, frozen, and later re-implanted after cancer treatment to restore ovarian function. Ovarian tissue is harvested laparoscopically before radiation exposure and stored at ultra-low temperatures until needed. This technique is the only option for pre-pubertal girls or women who cannot delay cancer treatment for oocyte retrieval. Although oocyte and embryo cryopreservation have higher success rates, ovarian tissue cryopreservation offers hope for restoring fertility in young cancer survivors. Improved cryopreservation techniques, such as vitrification, have significantly enhanced survival rates for oocytes and embryos. In addition, the development of artificial ovaries to support the maturation of ovarian tissue ex vivo is under investigation.[7,11-14,27]
GnRH agonists
The administration of GnRH agonists during radiotherapy helps protect ovarian function by reducing gonadotropin stimulation, effectively placing the ovaries in a dormant state and making them less susceptible to radiation damage. This strategy has shown promise, particularly in women undergoing chemotherapy.[13,25]
These strategies offer hope to women at risk of gonadotoxic exposure due to medical treatments. Integrating these methods with advances in radiotherapy, such as proton therapy, volumetric-modulated arc therapy (VMAT), and intensity-modulated radiation therapy (IMRT), enhances the ability to preserve fertility while ensuring effective treatment.[8]
Dose optimization in diagnostics
Adhering to the “As Low As Reasonably Achievable” principles in medical imaging protocols helps minimize radiation exposure. Ionizing radiation represents a dual-edged sword in medical care, offering diagnostic and therapeutic benefits but with the potential for serious reproductive harm. Awareness of its impacts and the adoption of protective measures are essential to safeguard the reproductive health of women exposed to radiation in both medical and occupational settings.[4,11,12,27]
Pre-treatment counseling and multidisciplinary approach
Pre-treatment counseling and a multidisciplinary approach involving oncologists, radiologists, and reproductive specialists are essential for the successful application of fertility preservation techniques. Collaborative planning ensures that women undergoing gonadotoxic treatments are informed about their options and supported in making decisions tailored to their reproductive goals.[4,11,12,25,27]
EFFECTS ON MALE REPRODUCTIVE HEALTH
The male reproductive system is highly sensitive to ionizing radiation, especially the testes, which are responsible for spermatogenesis and the production of testosterone. Radiation exposure can have immediate and long-term consequences, depending on the dose, duration, and frequency of exposure, as well as the individual’s age and health status.
Impairment of spermatogenesis
Spermatogenesis, the process of sperm production, is highly radiosensitive because it involves rapidly dividing germ cells in the testes. Even low doses of radiation (as little as 0.1–0.2 Gy) can temporarily impair spermatogenesis, leading to reduced sperm count (oligospermia) and motility (asthenospermia). A dose of 1–2 Gy can cause significant suppression of sperm production, with recovery depending on the individual’s baseline health and the radiation dose. Doses above 4–6 Gy can cause permanent damage to the germinal epithelium, leading to azoospermia (complete absence of sperm) and irreversible infertility. Repeated exposures, such as those from radiotherapy for testicular or pelvic cancers, can cumulatively disrupt spermatogenesis. Recovery of spermatogenesis, if possible, can take months to years after exposure, depending on the extent of damage. However, residual effects, such as reduced sperm quality, may persist.[17-24,37,38]
DNA fragmentation in sperm cells
Radiation induces oxidative stress and DNA damage in sperm cells, even at low doses. Reactive oxygen species generated during radiation exposure cause double-strand breaks in DNA. These damages can affect the genetic integrity of sperm, potentially leading to infertility, miscarriage, or genetic abnormalities in offspring. Sperm DNA fragmentation may not immediately manifest as infertility but can contribute to poor fertilization rates and embryonic development issues during assisted reproductive technologies (ARTs).[17-24,34,36]
Testicular fibrosis
High-dose radiation can cause structural changes in the testes, including testicular fibrosis, which involves the replacement of healthy tissue with scar tissue. Fibrosis reduces the capacity of the testes to produce sperm and testosterone, exacerbating infertility and hormonal imbalances. Damage to the Sertoli and Leydig cells, which support spermatogenesis and testosterone production, respectively, is a key contributor to fibrosis.[18-23,37]
Hormonal disruption
Radiation exposure can interfere with the hypothalamic-pituitary-gonadal (HPG) axis, affecting testosterone production. Acute effects occur when high doses temporarily suppress testosterone production, causing fatigue, reduced libido, and other symptoms of hypogonadism. Chronic effects ensue when persistent damage to Leydig cells, which produce testosterone, may result in long-term hormonal deficiencies requiring hormone replacement therapy.[17-24,35,37]
Temporary infertility
Low to moderate doses of radiation often cause reversible infertility. However, the recovery period varies and may take years.
Permanent infertility
High-dose exposures (>4 Gy) or cumulative effects of radiotherapy targeting pelvic cancers can lead to permanent infertility. This is especially critical in young males undergoing treatment for conditions such as testicular cancer, prostate cancer, or Hodgkin lymphoma.[1-4,17-24,37]
Dose thresholds for testicular damage
The sensitivity of the testes to radiation has been extensively studied, leading to well-defined dose thresholds:
0.1–0.5 Gy: Transient reduction in sperm count with recovery in weeks to months
1–2 Gy: Prolonged suppression of spermatogenesis, with recovery taking 1–3 years
>4 Gy: Permanent damage to spermatogenesis, resulting in infertility
>15 Gy: Severe damage to Leydig cells, leading to hypogonadism and permanent testosterone deficiency.[5,11,12,17-24,25-27,29,30,32-35]
Risk of genetic effects
Radiation-induced damage to sperm DNA can have intergenerational consequences. Damaged sperm may carry mutations that increase the risk of congenital anomalies in offspring. Radiation may alter the epigenetic profile of sperm, potentially affecting embryonic development and the health of future generations.[23]
The effects of graded doses of ionizing radiation was studied by Rowley et al. and the relation between recovery of spermatogenesis and graded doses is as shown in Table 1.[24]
| Radiation dose | Time to recovery |
|---|---|
| <1 Gy | 9–18 months |
| 2–3 Gy | 30 months |
| >4 Gy | >5 years |
Several approaches are employed to minimize radiation-induced damage to male reproductive health:
Testicular Shielding: Testicular shielding involves the placement of protective barriers, usually made of lead or other radiation-attenuating materials, over the testes during radiological procedures to block radiation exposure. The primary goal is to prevent scatter radiation from reaching the testes, reducing the risk of radiation-induced damage to the male reproductive organs.
In procedures such as X-rays, CT scans, and fluoroscopy, testicular shielding is commonly used when imaging the pelvic region. In cancer treatments involving radiation, especially in pelvic or abdominal regions (e.g., prostate or rectal cancers), testicular shielding can protect the testes from non-targeted radiation exposure.[4]
Gonadal shielding during total-body radiotherapy protects the germinal epithelium. Adolescent (and childhood) patients who did not have testicular shielding had a significantly smaller testicular volume in adulthood compared with those who received testicular shielding. Diminished testosterone/luteinizing hormone ratio was also reported without testicular shielding.[5,30,32-37]
In some cases, especially during pelvic radiotherapy, testicular shielding can interfere with the precision of the treatment if the shield inadvertently blocks part of the radiation field needed for tumor targeting. This requires careful planning and expert management. Shielding is effective in protecting the testes from scatter radiation but is not as effective when radiation is directly focused on the testes, which can occur in certain cancer treatments. Recent advances include the development of tailored shields that better fit individual anatomies, improving their effectiveness while minimizing interference with the treatment area. The integration of more advanced imaging techniques, such as CT and magnetic resonance imaging (MRI) scans, allows for personalized treatment plans and better placement of shields during radiotherapy, reducing unnecessary radiation exposure.[4-8]
Fertility preservation
Fertility preservation refers to the practice of collecting and storing sperm or other reproductive tissues before undergoing gonadotoxic treatments (such as chemotherapy or radiation therapy). This allows men to preserve their ability to father biological children in the future, even if they experience infertility due to cancer treatment.[5,30,32-38]
Cryopreservation of sperm
The most commonly used and effective method for men is sperm banking, where sperm is collected and frozen for future use in ARTs such as IVF. Sperm can be stored for years and thawed later to fertilize eggs.[11,12,17-24,25-27,39]
Ongoing collection
For men undergoing long-term treatment, sperm can be collected over time, ensuring a better chance of having viable sperm for future use.
Use of donor sperm
In cases where sperm collection is not possible, donor sperm may be an option for couples planning to have children.[40]
Young men, including those who have not yet reached reproductive maturity, may benefit from sperm banking before they undergo gonadotoxic treatments. Advances in freezing and thawing techniques, such as vitrification (a flash-freezing method), have increased the success rates of sperm survival and quality after thawing.[41] In cases where sperm collection is not feasible (e.g., prepubertal boys), testicular tissue can be harvested and frozen. This tissue may be re-implanted or used for sperm production in the future.[39]
Radioprotective agents
Radioprotective agents are compounds that help protect tissues from the damaging effects of ionizing radiation. These agents reduce oxidative stress and DNA damage caused by radiation exposure, thus preventing long-term cellular and genetic harm, particularly to sensitive tissues like the testes.[39-42]
Amifostine
One of the most studied radioprotective agents, amifostine, is a sulfur-containing compound that reduces the damage caused by radiation. It works by scavenging free radicals and preventing DNA damage in healthy tissues while allowing for the full therapeutic effect of radiation on tumor cells.[39,42]
Antioxidants
Other compounds, such as Vitamin C, Vitamin E, and glutathione, are antioxidants that help neutralize free radicals produced during radiation exposure. These agents may reduce oxidative damage to sperm DNA and testicular cells, decreasing the risk of infertility.[43-45]
Amifostine is sometimes used in cancer patients receiving radiation therapy to protect normal tissues (including the testes) from radiation damage. Its administration, however, requires careful timing and dosage to minimize side effects.[39-42]
Particularly in pelvic cancer treatments, where high doses of radiation are required, radioprotective agents can be used to protect reproductive organs like the testes from collateral damage. These agents may be used as an adjunct to fertility preservation strategies, helping to protect sperm production and function during cancer treatments.[40-42] Ongoing research into other potential radioprotective agents, including novel drugs and natural compounds, offers hope for improving fertility preservation in men undergoing gonadotoxic treatments. Advances in understanding the molecular mechanisms of radiation damage have led to the development of more targeted radioprotective therapies that can better preserve reproductive health while enabling effective cancer treatment.[39-45]
Advanced radiotherapy techniques
Advanced radiotherapy techniques focus on delivering precise, high-dose radiation to tumors while minimizing damage to surrounding healthy tissues, including the testes. These innovations have improved the ability to protect male reproductive health during cancer treatment, reducing the risk of infertility and other long-term effects.
Proton therapy
Proton therapy uses protons instead of X-rays to treat cancer. Because protons deposit their energy more precisely at the tumor site and have minimal radiation leakage beyond the target, they offer a significant advantage in sparing healthy tissues, including the testes. Proton therapy is particularly useful for tumors located near reproductive organs.[46]
Intensity-modulated radiotherapy (IMRT)
IMRT allows for the precise delivery of radiation beams to a tumor, adjusting the intensity of radiation at different points of the beam. This technique significantly reduces exposure to surrounding tissues, such as the testes, while ensuring that the tumor receives an effective dose of radiation.[46]
Image-guided radiotherapy (IGRT)
IGRT involves using imaging technology, such as CT scans, during radiotherapy to ensure the tumor is precisely targeted. This method helps minimize the irradiation of healthy tissues, including reproductive organs, particularly in pelvic radiation therapy.
IMRT and proton therapy are commonly used for cancers of the prostate, rectum, and other pelvic organs, where radiation often risks damage to the testes. These techniques are also used for cancers in the head and neck, where radiation to nearby organs, including the testes, needs to be minimized.
Advancements in real-time imaging and treatment planning software allow for better delineation of tumors and surrounding healthy tissues, leading to more effective protection of reproductive organs. Combining these advanced radiotherapy methods with sperm banking or other fertility-preserving measures offers an even more comprehensive approach to protecting male fertility during cancer treatment.
With continued advancements in medical technology and fertility preservation strategies, men undergoing cancer treatment now have better opportunities to preserve their fertility and protect their long-term reproductive health. Early identification of at-risk individuals and the implementation of protective strategies are essential to mitigate these effects and preserve fertility and hormonal function in men exposed to radiation in medical or occupational settings.
Diagnostic and therapeutic nuclear medicine procedures
Fertility issues following nuclear medicine diagnostic and therapeutic procedures depend on several factors, including the type of radiopharmaceutical used, the radiation dose received by reproductive organs, and the patient’s overall health and age.[47-49]
Diagnostic procedures
Most diagnostic procedures (e.g., positron emission tomography/CT, bone scans, and thyroid scans) involve small amounts of radioisotopes with relatively low radiation doses. The radiation dose to reproductive organs in these procedures is typically negligible and unlikely to impair fertility.[50] Women of childbearing age are often asked about pregnancy before diagnostic procedures to avoid fetal radiation exposure. Diagnostic procedures are generally postponed or substituted with non-ionizing imaging (e.g., ultrasound or MRI) if pregnancy is suspected.
Therapeutic Procedures: Therapeutic nuclear medicine procedures pose a higher risk. Therapeutic procedures (e.g., I-131 for thyroid cancer, Lu-177 DOTATATE for neuroendocrine tumors, or Y-90 for liver cancer) use much higher radiation doses, which could potentially impact fertility, especially if gonads are exposed.[41] Young individuals may be at higher risk due to the sensitivity of gonadal tissue. In women, high doses of radiation can damage ovarian reserve, leading to premature ovarian failure. In men, the testes are highly radiosensitive, and exposure may reduce sperm count or motility. Repeated treatments or high cumulative doses may increase the risk of infertility.
Minimizing gonadal exposure
Measures to minimize gonadal exposure include administering treatments in ways that minimize unnecessary radiation exposure. Recommended precautions are sperm/egg preservation for patients undergoing high-dose therapy. Sperm or egg banking is recommended before treatment to preserve reproductive potential. Patients are advised to use contraception during and for a recommended period after therapy to avoid potential radiation effects on gametes or a developing fetus.[4-8,39-47]
Radiation dose thresholds for ovaries and testes
Radiation dose thresholds for ovarian damage are significant in fertility considerations. Permanent sterility in women can occur at doses >6 Gy. For testes, temporary sterility can occur at doses of 0.1–2 Gy, with permanent sterility at doses >6 Gy. In men, spermatogenesis can recover after temporary suppression if the dose is within a reparable range, although recovery can take several months to years. In women, ovarian tissue does not regenerate, so damage is often irreversible.[11,12,17-24,25-27]
Fertility counseling and consultation
Patients should be informed of potential fertility risks before undergoing nuclear medicine therapy. Consultation with a fertility specialist is recommended for patients concerned about preserving reproductive potential.[5,30,32-38] This ensures that fertility preservation strategies, including sperm or egg banking, are considered early in the treatment process, especially for those at higher risk due to age or the nature of the therapy.
As per the American Society of Clinical Oncology’s recommendation for fertility preservation in people treated for cancer, Lee et al. have described and recommended the following strategy as produced in a Figure 1.[50]
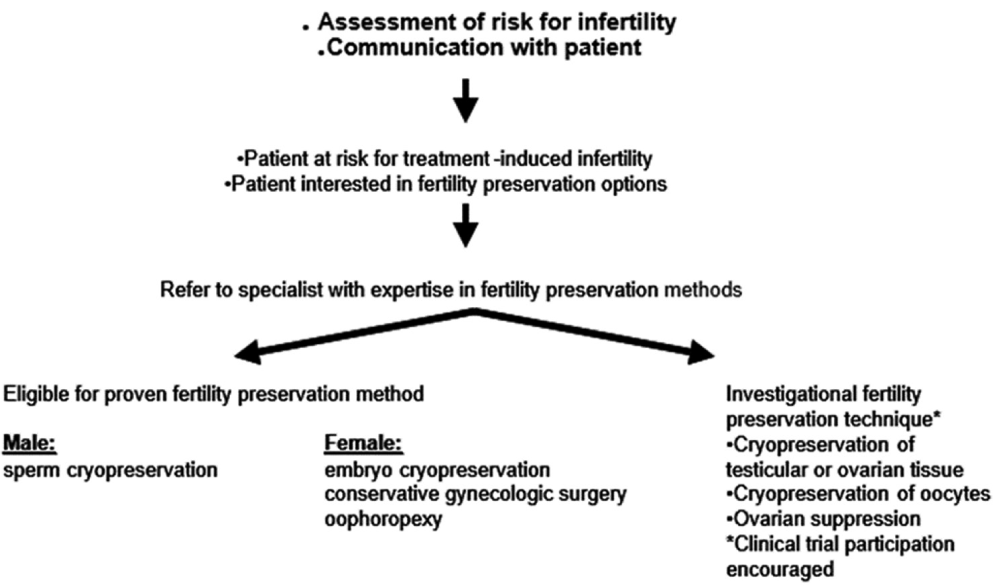
- Flow chart depicting ASCO recommendation for fertility preservation in people treated for cancer. ASCO: American Society of Clinical Oncology.
MITIGATION STRATEGIES FOR MEDICAL RADIATION EXPOSURE
Adherence to ALARA principles
The ALARA principle underscores the importance of minimizing radiation exposure in medical settings. Techniques such as dose optimization, patient shielding, and the judicious use of imaging are critical.[11-14,27]
Education and counseling
Raising awareness among healthcare providers and patients about the reproductive risks of ionizing radiation is vital. Pre-treatment counseling about fertility preservation options can empower patients to make informed decisions.[5,29,30,32,33,35]
Advances in radioprotective agents
Emerging research on radioprotective agents, such as amifostine and antioxidants, offers promising avenues for reducing radiation-induced reproductive damage. These agents work by scavenging free radicals and enhancing DNA repair mechanisms.[39-42]
CONCLUSION
The use of ionizing radiation in medical applications, while indispensable, necessitates a careful balance between therapeutic efficacy and the preservation of reproductive health. Advances in radiotherapy, such as IMRT and proton therapy, alongside fertility-preserving techniques, have significantly mitigated the risks associated with radiation exposure. Continued research, patient education, and adherence to safety protocols are essential to ensure that the benefits of medical radiation are maximized without compromising reproductive health. By integrating these strategies, healthcare providers can better support patients in achieving optimal outcomes both in their cancer treatment and long-term reproductive well-being.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Effect of radiation on the human reproductive system. Environ Health Perspect. 1993;101:109-16.
- [CrossRef] [PubMed] [Google Scholar]
- Ionizing radiation and reproductive health. Front Public Health. 2023;11:1147934.
- [CrossRef] [PubMed] [Google Scholar]
- Family size and duration of fertility in female cancer survivors: A population-based analysis. Fertil Steril. 2022;117:387-95.
- [CrossRef] [Google Scholar]
- Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902-11.
- [CrossRef] [PubMed] [Google Scholar]
- The radiosensitivity of the human oocyte. Hum Reprod. 2003;18:117-21.
- [CrossRef] [PubMed] [Google Scholar]
- Do cancer therapies damage the uterus and compromise fertility? Hum Reprod Update. 2020;26:161-73.
- [CrossRef] [PubMed] [Google Scholar]
- Hot topics on fertility preservation for women and girls-current research, knowledge gaps, and future possibilities. J Clin Med. 2021;10:1650.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73:1304-12.
- [CrossRef] [PubMed] [Google Scholar]
- The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409-25. Erratum in: Nat Rev Cancer 2015;15:509
- [CrossRef] [PubMed] [Google Scholar]
- Ovarian transposition by laparoscopy before radiotherapy in the treatment of Hodgkin's disease. Cancer. 1998;83:1420-4.
- [CrossRef] [Google Scholar]
- Cancer and fertility preservation. J Clin Oncol. 2005;23:2651-7.
- [CrossRef] [PubMed] [Google Scholar]
- Fertility preservation in female cancer patients: An overview. J Hum Reprod Sci. 2015;8:3-13.
- [CrossRef] [PubMed] [Google Scholar]
- Fertility preservation in women undergoing cancer treatment. Hum Reprod Update. 2004;10:635-43.
- [CrossRef] [PubMed] [Google Scholar]
- Female oncofertility: Current understandings, therapeutic approaches, controversies, and future perspectives. J Clin Med. 2021;10:5690.
- [CrossRef] [PubMed] [Google Scholar]
- Update on fertility preservation for younger women with breast cancer. CMAJ. 2020;192:E1003-9.
- [CrossRef] [PubMed] [Google Scholar]
- Fertility and pregnancy outcome after abdominal irradiation that included or excluded the pelvis in childhood tumor survivors. Int J Radiat Oncol Biol Phys. 2010;76:867-73.
- [CrossRef] [PubMed] [Google Scholar]
- Electroejaculation as a method of fertility preservation in boys diagnosed with cancer: A single-center experience and review of the literature. Fertil Steril. 2014;102:199-205.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Radiations and male fertility. Reprod Biol Endocrinol. 2018;16:118.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of radiofrequency exposure on male fertility: A systematic review of human observational studies with dose-response meta-analysis. Environ Int. 2024;190:108817.
- [CrossRef] [PubMed] [Google Scholar]
- Association between reproductive health and nonionizing radiation exposure. Electromagn Biol Med. 2021;40:92-102.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of chemotherapy and radiation on spermatogenesis in humans. J Natl Cancer Inst Monogr. 2013;46:13-8.
- [Google Scholar]
- Testicular function following chemotherapy. Hum Reprod Update. 2001;7:363-9.
- [CrossRef] [PubMed] [Google Scholar]
- A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of graded doses of ionizing radiation on the human testis. Radiat Res. 1974;59:665-78.
- [CrossRef] [PubMed] [Google Scholar]
- Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: A meta-analysis of randomized studies. Ann Oncol. 2015;26:2408-19.
- [CrossRef] [PubMed] [Google Scholar]
- Fertility preservation in female cancer patients. ISRN Obstet Gynecol. 2012;2012:807302.
- [CrossRef] [PubMed] [Google Scholar]
- Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2020;31:1664-78.
- [CrossRef] [PubMed] [Google Scholar]
- Fertility preservation in adult male cancer patients. Cancer Treat Res. 2007;138:28-49.
- [CrossRef] [PubMed] [Google Scholar]
- Fertility preservation for young patients with cancer: Who is at risk and what can be offered? Lancet Oncol. 2005;6:209-18.
- [CrossRef] [PubMed] [Google Scholar]
- Male reproductive health after childhood, adolescent, and young adult cancers: A report from the Children's Oncology Group. J Clin Oncol. 2012;30:3408-16.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic preservation of ovarian function: An underused procedure. Am J Obstet Gynecol. 2003;188:367-70.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy after adolescent and adult cancer: A population-based matched cohort study. Int J Cancer. 2011;129:1225-36.
- [CrossRef] [PubMed] [Google Scholar]
- Human ovarian reserve from conception to the menopause. PLoS One. 2010;5:e8772.
- [CrossRef] [PubMed] [Google Scholar]
- Gonadal status and reproductive function following treatment for Hodgkin's disease in childhood: The Stanford experience. Int J Radiat Oncol Biol Phys. 1990;19:873-80.
- [CrossRef] [PubMed] [Google Scholar]
- Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breat cancer. J Clin Oncol. 2015;33:2424-9.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of ovarian transposition in gynaecological cancers; a systematic review and meta-analysis. J Ovarian Res. 2014;7:69.
- [CrossRef] [PubMed] [Google Scholar]
- Gonadal shielding to irradiation is effective in protecting testicular growth and function in long-term survivors of bone marrow transplantation during childhood or adolescence. Bone Marrow Transplant. 2007;39:483-90.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of radiation on male fertility. Adv Exp Med Biol. 2022;1391:71-82.
- [CrossRef] [PubMed] [Google Scholar]
- An overview on ethical issues about sperm donation. Asian J Androl. 2009;11:645-52.
- [CrossRef] [PubMed] [Google Scholar]
- Sperm cryopreservation for impaired spermatogenesis. Reprod Fertil 2022:4F-0106.
- [CrossRef] [PubMed] [Google Scholar]
- Radioprotectors, radiomitigators, and radiosensitizers In: Baatout S, ed. Radiobiology textbook. Cham: Springer; 2023. p. :171-86.
- [CrossRef] [Google Scholar]
- Natural guardians: Natural compounds as radioprotectors in cancer therapy. Int J Mol Sci. 2024;25:6937.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of radiotherapy on antioxidant vitamin E in patients with carcinoma uterine cervix-a pilot study. J Oncol Cancer Res. 2017;1:1-7.
- [CrossRef] [Google Scholar]
- Relevance of serum Vitamin C levels in cancer patients undergoing radiotherapy treatment. J Gynecol Oncol. 2021;4:1055.
- [Google Scholar]
- The predicted relative risk of premature ovarian failure for three radiotherapy modalities in a girl receiving craniospinal irradiation. Phys Med Biol. 2013;58:3107-23.
- [CrossRef] [PubMed] [Google Scholar]
- Radiation in medicine: Origins, risks and aspirations. Glob Cardiol Sci Pract. 2014;2014:437-48.
- [CrossRef] [PubMed] [Google Scholar]
- Nuclear imaging of a pregnant patient: Should we perform nuclear medicine procedures during pregnancy? Mol Imaging Radionucl Ther. 2012;21:1-5.
- [CrossRef] [PubMed] [Google Scholar]
- Radiation dose and risks to fetus from nuclear medicine procedures. Phys Med. 2017;43:190-8.
- [CrossRef] [PubMed] [Google Scholar]
- American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-31. Erratum in: J Clin Oncol 2006;24:5790
- [CrossRef] [PubMed] [Google Scholar]







