Translate this page into:
Quantum imaging-based nanobiosensors: Pioneering point-of-care approach for early diagnosis of environmental-linked breast cancer

*Corresponding author: Pradyumna Kumar Mishra, Division of Environmental Biotechnology, Genetics and Molecular Biology, ICMR-National Institute for Research in Environmental Health, Bhopal, Madhya Pradesh, India. pkm_8bh@yahoo.co.uk
-
Received: ,
Accepted: ,
How to cite this article: Ratre P, Thareja S, Mishra PK. Quantum imaging-based nanobiosensors: Pioneering point-of-care approach for early diagnosis of environmental-linked breast cancer. J Reprod Healthc Med. 2024;5:9. doi: 10.25259/JRHM_10_2024
Abstract
Early detection is paramount for successful treatment outcomes in cancer diagnosis. Among women across the globe, breast cancer (BC) ranks as one of the deadliest forms of cancer. Prolonged exposure to numerous environmental pollutants has been linked to epigenetic reprogramming, which entails changes in the expression patterns of non-coding RNAs. These alterations have been strongly linked to an increased risk of developing BC. Women are confronted with hazardous smoke from polluting stoves and fuels for longer as they often perform home duties such as cooking. Inefficient combustion emits black carbon (sooty particles), which enters the bloodstream and is strongly connected to an elevated risk of BC. The use of several analytical methods, such as real-time polymerase chain reaction, microarray, and sequencing, has numerous disadvantages, such as high expenses, limitations in sensitivity, and lack of accuracy. However, the emergence of quantum dots (QDs), nanoscale semiconductor particles with unique optical properties, and the development of quantum imaging-based sensors offer a glimpse into the future of medical technology. These sensors have the potential to completely change the medical field by offering highly precise, non-invasive, and reliable techniques for early diagnosis. Our article delves into the intricacies of QDs imaging-based sensors, their applications in BC detection, and their transformative impact on improving patient care. In recent years, the confluence of quantum science and diagnostic imaging has opened new avenues for BC diagnosis. The present state of quantum imaging-based BC diagnosis sensors is examined in this article, along with potential future developments with the help of artificial intelligence.
Keywords
Nanobiosensor
Circulating epigenomic signature
Artificial intelligence
Environmental health
Translational research
INTRODUCTION
Breast cancer (BC) continues to be one of the most major and concerning health issues worldwide, affecting millions of women every year. In recent decades, the rapid industrialization and urbanization of societies have led to unprecedented levels of environmental pollution. From air pollutants released by vehicles and industries to chemicals leaching into water sources and soil, humans are increasingly exposed to a cocktail of harmful substances. Among the myriad health risks posed by environmental pollution, its association with BC has garnered significant attention from researchers and public health experts alike. While genetics and lifestyle choices play significant roles in its development, emerging research highlights the profound impact of household air pollution (HAP) and solid fuels (SFs) on BC risk.[1] Open flames or inefficient stoves operated by coal, kerosene, and biomass (wood, animal dung, and agricultural waste) are used by around 2.3 billion people throughout the globe, or about a third of the world’s population, for cooking purpose. This leads to dangerous air pollution in homes. In 2020, almost 3.2 million people died each year as a result of HAP, with more than 237,000 of the casualties being children <5 years old. An estimated 6.9 million people die too soon each year as a result of air pollution in their homes and the surrounding environment. By 2030, it is anticipated that around 2.1 billion people will still not have access to clean fuels and technology unless there is significant regulation.[2] In underdeveloped countries such as India, around 64% of homes use SF as their main and backup source of cooking fuel, particularly rural households utilizing it at a far greater proportion (81%) than urban households (26%). Brimming SF is simpler and less expensive than using electric or LPG, particularly for those who live in remote locations.[3] These fuels are frequently not burned completely in homes due to basic, poorly constructed stoves that lack an exhaust chimney to remove pollutants. Therefore, when ventilation is insufficient, a variety of hazardous pollutants are generated in large quantities, including heavy metals such as cadmium, arsenic, lead, fluorine, and vanadium, as well as polycyclic aromatic hydrocarbons (PAHs), volatile organic compounds (VOCs), particulate matter (PM), sulfur dioxide (SO2), carbon monoxide (CO), nitrous oxide (NO), black carbon, and chlorinated dioxins. They have a substantial risk of endangering human health.[4,5] This article delves into the intricate relationship between HAP and BC, exploring the various HAP pollutants implicated, their mechanisms of action, and strategies for mitigation and prevention.
According to earlier research, diagnosing early BC combined with appropriate treatment may considerably lower the long-term mortality risk from BC. For BC treatment to be effective, early diagnosis is essential, as it is for other malignancies. If the disease has started advancing, selecting appropriate therapy involves information relating to the expression of gene profile, mutations, and polymorphisms, alongside the secretome and proteome of tumor cells and their milieu.[6] The present gold-standard method for breast screening and imaging, mammography, likely to be less sensitive to smaller tumors (<1 mm, or around 100,000 cells), is not as efficient in patients under 40 and is unable to predict how the illness may progress. Another method, contrast-enhanced digital mammography provides more accurate diagnostic results than mammography and ultrasonography in cases with dense breast tissue, but widespread availability is constrained by its high radiation exposure and high cost. As an extramedical imaging method to mammography, ultrasound has been employed. Another technique, magnetic resonance imaging (MRI), can identify small lesions that are not detected by mammography.[7] However, MRI may be overdiagnosed due to its limited sensitivity and high cost. For the most precise tool for evaluating how tumors spread and respond to therapy, positron emission tomography is a useful technique.[8]
The field of modern nanomedicine has enormous potential to advance both therapeutic medication delivery and cancer diagnostics. Due to their increased permeability and retention impact, nanoparticles (NPs) without particular target functionalities might accumulate in molecularly diverse tumors. NPs are flexible diagnostic scaffolds due to their extensive surface modification and incorporation with targeted capabilities, medications, and external imaging probes; nonetheless, the rate of clinical translation depends critically on the NPs’ rational and simple design.[9,10] To further reduce possible toxicity, the urinary system’s ability to remove NPs is a very desirable characteristic. A key component of nanotechnologies is optical-based NPs imaging, including quantum dots (QDs)-based imaging, that recently demonstrated intriguing prospective possibilities for cancer research. Compared with conventional organic dyes, QDs exhibit numerous superior optical characteristics. These include resilient resistance to photobleaching, high fluorescence intensity and chemical oxidation, size-tunable emissions wavelength, broad two-photon cross-section, and concurrent various fluorescence for one excitation source. The majority of the QDs tend to be semiconductor nanocrystals. QDs-based imaging has become widely used in cancer research due to its advantages in optics. Concentrating on potential clinical uses, this review outlines the level of QDs-based imaging in BC research and its future directions.[11]
COMPREHENDING ENVIRONMENTAL AIR POLLUTION
Environmental pollutants linked to BC encompass a wide array of substances, each with its unique mechanisms of action. The constituents of air pollution include carcinogenic substances, which have the ability to enhance oxidative stress or cause DNA damage, as well as possess qualities that alter the endocrine system. One prominent group is endocrine-disrupting chemicals (EDCs), which interfere with hormonal balance in the body. Compounds such as bisphenol A, commonly found in plastics, and phthalates, used in personal care products, have been implicated in BC development.[12] These chemicals mimic or block the actions of natural hormones, disrupting normal physiological processes and potentially fuelling cancer growth. Another concerning category is persistent organic pollutants (POPs), which include pesticides, industrial chemicals, and byproducts of combustion. Examples include polychlorinated biphenyls (PCBs), dioxins, and certain pesticides such as dichlorodiphenyltrichloroethane (DDT).[13] These compounds accumulate in the environment and the food chain, posing long-term health risks. Research suggests that exposure to POPs may alter gene expression, promote inflammation, and contribute to the initiation and progression of BC. Air pollution, a ubiquitous problem in many urban areas, has also been linked to BC risk. PM, ozone, NO, and VOCs are among the harmful components of air pollution.[14] These pollutants can induce oxidative stress, trigger inflammation, and disrupt cellular signaling pathways, all of which are implicated in cancer development. In addition, certain air pollutants may act as carcinogens, directly damaging DNA and increasing the likelihood of malignant transformation. Indoor air pollution, often overlooked compared to outdoor pollution, is also recognized as a significant risk factor for various health conditions, including BC.[15]
Environmental pollutants are often encountered in daily life, stemming from chemicals present in consumer goods, air and water pollution, food contamination, and workplace exposure for women. Lately, a limited number of research initiatives have shown evidence, both direct and indirect, linking ambient air pollution to BC. Two American studies have looked at the relationship link air pollution and survivability after being diagnosed with BC. Figures of PM <10 µm (PM10) and PM2.5 had a significant connection with greater BC deaths in an analysis of 255,128 women diagnosed with BC according to statistics collected by the Surveillance, Epidemiology, and End Results program. More robust associations were observed for localized disease. Assessments of PM10 and PM2.5 derived from 2-year means were not linked with BC death in the 8936 patients of the Nurses’ Health Study. However, there was a statistically significant link with PM2.5 among individuals with stage I illness.[16] Another study revealed promising results for a yearly PM approximation and all-cause mortality between estrogen receptor (ER) positive BCs, whereas an Italian investigation found a correlation between BC mortality and PM2.5 measured over a median of 3 years after diagnosis. These last two investigations, however, did not take lifestyle variables into account.[17]
The precise mechanism by which gaseous and PM air pollution may impact the development of BC remains unknown. Exposure to nitrogen oxides and nitrogen dioxide (NO2) is considered an indicator of exposure to air pollutants from traffic, which is a complicated combination of various PAHs, benzo (a) pyrene, benzene, metals, and other substances. PAHs are well-documented properties that may cause changes in DNA (mutagenic) and lead to the development of cancer. Studies have shown that PAHs can induce the formation of mammary tumors in laboratory rodents. The exposure to PAHs from traffic may elevate the risk of breast tissue damage by promoting the production of PAH-DNA adducts. The Long Island BC Study discovered an elevated susceptibility to BC in connection with PAH-adducts in blood lymphocytes, with traffic pollution being only one of the several sources of PAHs. Methylation is a possible biological process that may be involved in the association between PAH sources and changes in methylation levels at various promoter regions in BCs and in the blood of control women.[18]
There needs to be more investigation into the link connecting air pollution and death in BC, with appropriate adjustments made for individual-level variables. Furthermore, studies are necessary to determine whether distinct death risks are related to susceptibility to air pollution experienced by specific racial and socioeconomic classes who frequently live in locations with greater degrees of air pollution. Apart from ambient air pollutants, a high number of BC cases are also caused by indoor air pollution.
INDOOR AIR POLLUTION’S LINK TO BC UNDERSCORES SIGNIFICANT HEALTH CONCERNS
SF combustion, in many developing countries, indoor air pollution primarily stems from the burning of SFs such as wood and coal cooking and heating purposes. These combustion processes release a cocktail of harmful pollutants, including PM, VOCs, PAHs, and CO, all of which can pose health risks. Indoor air pollutants from household products, building materials, and furnishings can also contribute to BC risk. Chemicals such as formaldehyde, benzene, and phthalates, commonly found in paints, adhesives, plastics, and cleaning agents, have been linked to hormone disruption and carcinogenesis. Exposure to tobacco smoke indoors, known as secondhand smoke (SHS), is a well-established risk factor for various cancers, including BC. SHS contains numerous carcinogens and toxic chemicals, which can exert their harmful effects on both smokers and non-smokers alike.[19] Radon, a naturally occurring radioactive gas released from the decay of uranium in soil and rocks, can seep into buildings and accumulate indoors. Prolonged exposure to elevated levels of radon has been associated with an increased risk of lung cancer, but emerging evidence suggests a potential link to other cancers, including BC.[20] Cooking activities can generate indoor air pollutants, particularly when using high-temperature cooking methods or frying foods. Cooking oil fumes contain PAHs, heterocyclic amines, and other carcinogens, which can be inhaled or absorbed through the skin, potentially increasing BC risk. Inadequate ventilation and poor building design can exacerbate indoor air pollution by trapping pollutants indoors and limiting fresh air exchange. Buildings with insufficient ventilation may have higher concentrations of indoor pollutants, increasing occupants’ exposure and related health risks. Many of the mentioned indoor air pollutants, including VOCs and phthalates, have endocrine-disrupting properties, meaning they can interfere with hormonal balance in the body [Table 1].[24-38] Hormonal disruptions, particularly alterations in estrogen metabolism, have been implicated in BC development and progression.[21] A systematic review by Zeinomar et al. in 2020, demonstrates an epidemiological literature of environmental exposures and BC risk.[22] The authors conducted a comprehensive analysis of the existing literature on the correlation between environmental chemical exposures (ECE) and BC. Specifically, they focused on three kinds of research or subgroup analyses that specifically examined individuals with a greater absolute risk of developing BC . The selected subgroups for the study are family history of BC (Type 1), early-onset BC (Type 2), and genetic predisposition (Type 3). They reviewed 100 papers across 56 epidemiological studies. Only 3.6% included family history data, and 11% focused on early-onset BC. Among ten publications from eight studies, 80% found a significant link between (ECE) and BC risk. Factors studied included PAH, indoor cooking, NO2, DDT, PCBs, perfluorooctane sulfonamide (PFOSA), metals, personal care products, and industrial dyes. In Type 3 publications, 74% showed significant associations between PAHs, traffic-related pollution, PCBs, phthalates, PFOSAs, and genetic susceptibility markers related to carcinogen metabolism, DNA repair, oxidative stress, apoptosis, and tumor suppression pathways.[22]
| S. No. | Countries | Household air pollution sources and pollutants | Sample size | Conclusion | References |
|---|---|---|---|---|---|
| 1. | USA | Wood and synthetic logs (PAHs) | 1556 control 1508 cases | Studies have shown that the usage of synthetic logs for burning purposes can contribute to an increased risk of developing BC in women. | [24] |
| 2. | USA | Synthetic logs (PAHs) | 1556 control 1508 cases | Studies have revealed that indoor sources of PAHs have been associated with a rise of 30–50% in the occurrence of BC. This emphasizes the importance of identifying and controlling indoor sources of PAH to minimize the risk of developing BC. | [25] |
| 3. | USA | Wood (PAHs) | 50,884 Control 2416 cases | Employing an indoor wood-burning stove/fireplace at an extended adult residence at least thrice a week and heating either wood or natural gas/propane was linked to a slightly increased risk of BC. | [26] |
| 4. | Canada | PAHs | 1169 control 1130 cases | Long-term inhalation of PAHs may increase the risk of developing BC. | [27] |
| 5. | China | Biomass | 236,116 Cases | Burning solid fuel for cooking can raise a postmenopausal woman’s risk of death from cervical cancer and BC. | [28] |
| 6. | China | Organic dust extracts | - | An extract from organic indoor dust causes BC cells to become estrogen-responsive. | [29] |
| 7. | France | PM, NO, BAP, cadmium | 5222 controls 5222 cases | The study gives information on the risk of BC and prolonged exposure to specific air pollutants. | [30] |
| 8. | China | Biomass | 290,396 BC | Burning wood or coal for a long time may increase the BC risk. | [31] |
| 9. | USA | Dioxins and ambient PCDD/F | 35,908 BC cases | The data indicate that higher levels of exposure to PCDD/F pollutants in residential areas may be linked to a greater likelihood of developing BC. This investigation provides more evidence of a positive correlation at lower concentrations of air dioxin exposure. | [32] |
| 10. | New York City | PAHs | Daughters=186 and mothers=175(Prospective cohort study) | Exposure to combined levels of ambient Σ8 PAH and cigarette smoke throughout pregnancy may have a synergistic effect on the development of BTC in both mothers and daughters. | [33] |
| 11. | European Union | Smoking and second-hand smoke | - | Denmark, Malta, Croatia, Hungary, and the United Kingdom had the greatest burden in terms of both DALYs and fatalities caused by smoking and inhalation of SHS. The percentage of total BC DALYs and deaths in these countries was more than 4% and 5%, respectively. The countries with the lowest load, projected to be<1.5%, were Cyprus, Lithuania, Latvia, Italy, and Estonia. | [34] |
| 12. | France | Xenoestrogen Ambient Air Pollutants | 5222 invasive BC cases | It has been suggested that the correlation between pollutants and BC changes with estrogen receptor condition, as well as that the relationship may be modified by menopausal state. | [30] |
| 13. | China | Indoor dust | House dust showed lesser cytotoxicity but greater estrogenic potential on MCF-7 cells, whereas organic dust extract affected cell survival, changed the cell cycle, elevated intracellular Ca2+levels, and triggered cell cycle regulation and estrogen-responsive gene activities. | [29] | |
| 14. | California | Indoor and outdoor air, including particulates and endocrine disruptors | 1000 workers | According to this research, cumulative air pollution loads in an urban industrial environmental justice neighborhood were shown to be higher indoors than outdoors when compared to a rural community. | [35] |
| 15. | U.S. and Puerto Rico | Tobacco, marijuana, and indoor cooking sources in relation to AMH | 50,884 | Decreased AMH levels were linked to interior heating sources such as wood burning or the use of synthetic firelogs a minimum of 10 times per year in a domestic indoor stove. Women who smoked at least 20 cigarettes per day had significantly lower AMH levels compared to non-smokers, with a decrease of 56.2%. | [36] |
| 16. | Afghanistan | Household tobacco smoke | - | The death rate from BC was much higher in rural households (2.91; 1.02, 8.25). | [37] |
| 17. | Israel | Household low-frequency EMF | 1290 clinical case | EMF had a statistically significant impact on the development of every epithelial mammary tumor that was discovered. Invasive ductal carcinomas, probably the most prevalent kind of cancer in older women, are the ones where this impact is most noticeable. | [38] |
BPA: Bisphenol A, NO: Nitrous oxide, PAHs: Polycyclic aromatic hydrocarbons, PM: Particulate matter, PCDD/F: Polychlorinated dibenzo-p-dioxins and dibenzofurans, BTC: Breast tissue composition, DALYs: Disability-adjusted life years, SHS: Secondhand smoke, MCF-7: Michigan cancer foundation-7, AMH: Anti-Müllerian hormone, EMF: Electromagnetic fields, BaP: Benzo (a) pyrene, BC: Breast cancer.
They may also act synergistically with other risk causes, such as genetic predisposition, lifestyle factors, and external exposures, to increase BC risk. The cumulative impact of several contaminants and risk factors can amplify their individual impacts on cellular processes and cancer pathways. Implementing and enforcing regulatory policies aimed at reducing indoor air pollution, such as smoke-free laws, building codes, and emission standards for consumer products, are essential for protecting public health and minimizing cancer risks.[23] Although the effects of IAP on healthcare are becoming more well recognized, there are still gaps in our knowledge of how specifically it contributes to BC risk. Further research is needed to elucidate the mechanisms underlying indoor pollutants’ carcinogenic effects and to identify effective strategies for prevention and intervention.[24-28]
EXPLORING HOW INDOOR AIR POLLUTION CONTRIBUTES TO BC: MECHANISMS AND EVIDENCE UNVEILED
The mechanisms through which environmental pollutants are risk factor to BC are multifaceted and complex. At a cellular level, these substances can disrupt normal hormone signaling pathways, leading to unregulated cell division and growth. In addition, it has been demonstrated that most human breast tumors overexpress ERα, indicating that estrogen can cause these tumors to spread. Due to this, the signaling pathway is particularly vulnerable to EDCs that influence the effect of estrogen.[39] According to a recent investigation, 22 genes linked to the upstream regulator variables of ER 1 in Michigan cancer foundation-7 cells can be regulated by tri-o-cresyl phosphate, a kind of organophosphate ester frequently used as a plasticizer. This led to cell cycle disruption, stimulation of angiogenesis and the provision of nutrients, promoting tumor development, and assisting in tumor invasion and metastasis. Therefore, there may be a higher chance of BC if these EDCs combine with flame retardants.[40,41] Significant air pollutants, toxic gases, and SO2 are some of the other main components of biomass fumes. Overall, SO2 is regarded as a co-carcinogen. It may cause oxidative damage. Inhaled SO2 may destroy DNA, create chromosomal aberrations, and trigger mutations in several different genes. A study showed that breathing in SO2 was linked to harm to DNA in the respiratory tract and other organs, and it has been proposed that these damages may cause mutations and cancer.[42] Through a few common environmental activities, PAHs have been discovered to raise the risk of BC. They may be extracted from partially burnt wood and hot cooking oil. Following metabolic activation, PAHs transform into diolepoxides, which chemically bind to DNA to produce flexes in breast tissues. The findings of the study demonstrate the fact that metabolites of PAHs derived from cytochrome-P450 stimulate a variety of oxidative and electrophilic signaling mechanisms in cells and have a significant effect on cellular performance and govern epigenetic processes, particularly those related to DNA methylation and microRNAs (miRNAs) expressions.[43] Moreover, environmental pollutants can induce genetic mutations, epigenetic alterations, and oxidative damage, all of which are hallmarks of carcinogenesis. Beyond direct effects on breast tissue, environmental pollutants can modulate the tumor microenvironment, creating a favorable milieu for cancer progression. Chronic inflammation, a common consequence of pollutant exposure, can stimulate angiogenesis (the formation of new blood vessels) and enhance tumor invasion and metastasis. Furthermore, pollutants may impair immune function, compromising the body’s ability to detect and eliminate cancerous cells. In the area of epigenetics, aberrant miRNA expression is linked to the events that lead to the occurrence of cancer and could also play a role in the initiation of other diseases, including respiratory and cardiovascular conditions.[44,45] A research demonstrated a correlation between elevated blood miRNA levels and prolonged exposure to PM 2.5 (PM with a diameter <2.5 µm).[46] Subsequent investigation into the interaction among the intake of PM and miRNA gene expression in peripheral blood leukocytes demonstrated a correlation between PM exposure and the expression of miRNAs such as miR-126, miR-135a, miR-146a, miR-155, miR-21, miR-222, and miR-9.[47] Polymorphisms in the RNA processing genes Gem Nuclear Organelle Associated Protein 4 and DiGeorge Syndrome Critical Region 8 were shown to have an impact on the link between the expression of miRNAs and PM exposures.[48] These changes in non-coding RNAs (ncRNAs) expression after exposure to different pollutants can be detected in by QDs based designed nanosensors [Figure 1].
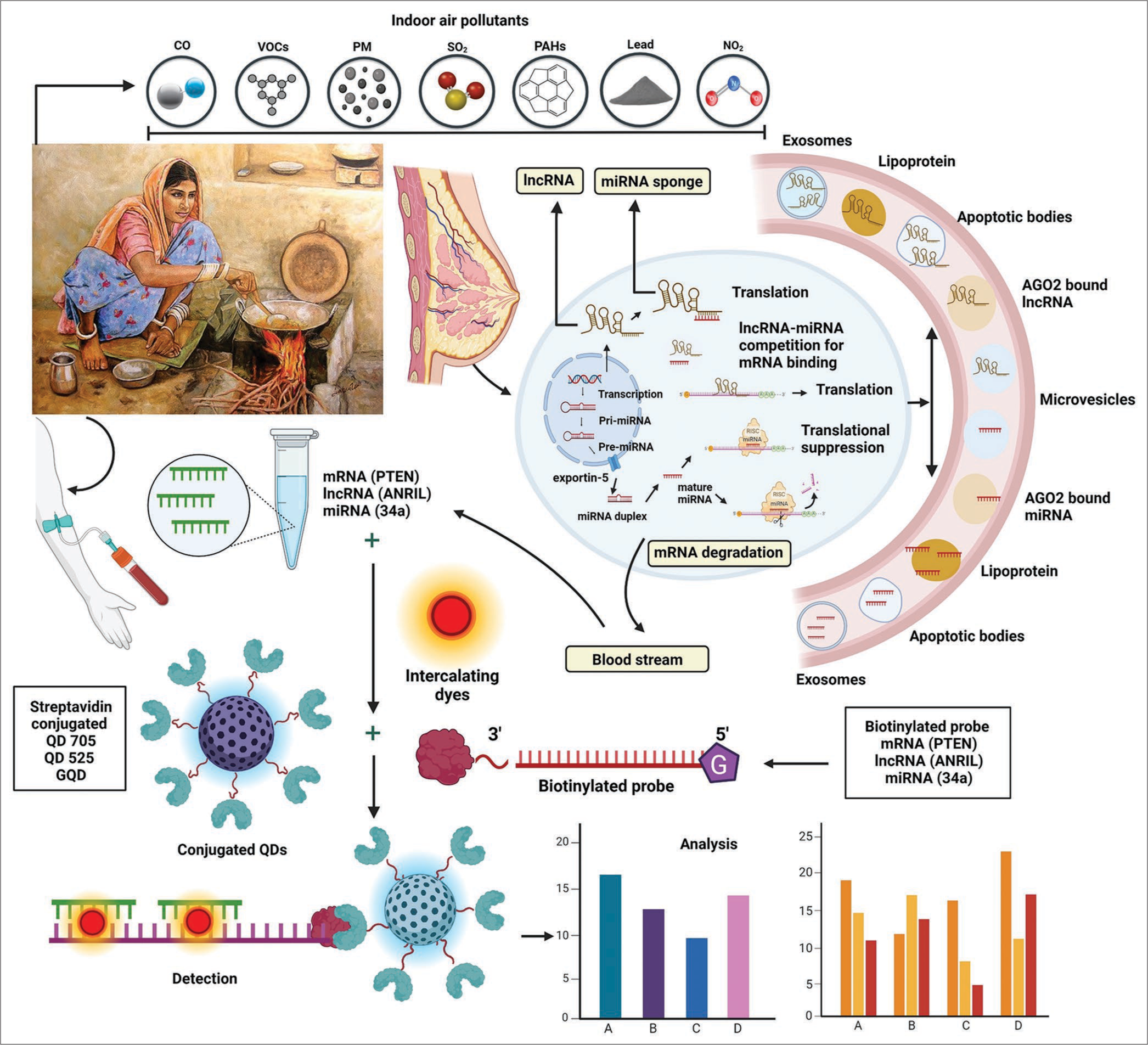
- The figure illustrates how exposure to various biomass smoke pollutants, including carbon monoxide (CO), volatile organic compounds (VOC), particulate matter (PM), nitrogen dioxide (NO2), sulfur dioxide (SO2), polycyclic aromatic hydrocarbons (PAH), and lead, causes abnormal expression of microRNA, long non-coding RNA, and messenger RNA in peripheral circulation. These biomarker aberrations are then detected by preparing quantum dot nanoconjugates and analyzing the results using various optical and quantum imaging techniques. AGO2: Argonaute protein 2, PTEN: Phosphatase and TENsin homolog deleted on chromosome 10, ANRIL: Antisense noncoding RNA in the INK4 Locus.
The link between environmental pollution and BC risk has been the subject of several epidemiological research, which have provided important insights into this intricate interaction. Although results differ between research and groups, there is a growing body of data pointing to a consistent relationship between several contaminants and a higher risk of BC. For example, a meta-analysis that was published in 2019 discovered a strong correlation between the prevalence of BC and exposition to air pollution, especially in postmenopausal women. Similarly, studies investigating the impact of POPs on BC risk have reported consistent associations, although with changes in specific chemicals and study populations. Consumption of organochlorine pesticides, including DDT, was shown to be linked to an elevated risk of BC by a critical assessment and meta-analysis that appeared in 2020. This finding emphasizes the significance of addressing environmental pollutants in cancer prevention initiatives. A study conducted in 15 European countries concerning 74,750 population to check the effect of outdoor (PM2.5 and10, NO2, and CO2) found that BC was found to have positive but statistically trivial links with PM2.5, PM10, PM coarse, and NO2 exposure.[49] Another one from China studied the effect of Environmental tobacco smoke in 23,415 participants (8388 men, 15,027 women) and discovered that ETS exposure was linked to a higher risk of cancer and all smoking-related malignancies through this population-wide prospective cohort analysis. However, the relationship was only marginally significant for lung cancer.[50] In the USA, a total of 2747 population was selected to test the effect of NO2 and PM, and by looking at the composition of PM2.5 component clusters, they found significant variation in the relationship between age acceleration and PM2.5 air pollution intake. All things considered, these results lend credence to the link connecting air pollution and genomic age, a measure of mortality and susceptibility to chronic illnesses.[51]
NANOBIOSENSORS: TRANSFORMING BC DIAGNOSTICS WITH CUTTING-EDGE TECHNOLOGY
Nanobiosensors are systems for analysis at the level of the nanoscale which employs biological materials coupled to the nanoscale as a transducing device to detect minuscule amounts of any analytes, whether it is biological, chemical, or physical. The required information is sent by the constructions using optical, electrochemical, thermometric, piezoelectric, magnetic, or micromechanical entails.[52] The notion of specific bio-recognition of cytoplasmic or surface biomarkers linked with cancer cells by coupled antibodies or bio-ligands is exactly what drives the produced outputs. Frameworks that use optical modules for detection have seen a sharp increase in prominence among the many kinds of sensing mechanisms due to their special qualities and benefits, which include improved sensitivity, simplicity in multiplexing, and adaptability.[53] By combining biological interactions that take place at the nanoscale range with plasmonic alterations in the transduction system’s product, optical biosensing enables the recognition of the macro-visual signals that are produced. Thus, using the complementary fields of molecular science and nanophotonics to develop next-generation point-of-care (POC) biosensing systems is an exciting prospect. Point-of-care testing (POCT) constitutes one of its most significant uses of biosensors. POCT involves conducting a diagnostic or prognostic test close to the patient to deliver speedy results. This means that the examination itself must be simple and quickly executed without needing pricey or sophisticated equipment. There is a strong need for biosensor systems that are sensitive, durable, portable, and affordable for clinical use for diagnosing diseases and monitoring therapy in POC contexts. Lateral-flow devices are well-established platforms for POC testing since they possess the majority of the aforementioned qualities of an excellent POC platform. A biosensor in POC applications consists of a transducer connected to a recognition device. The recognition event is then transformed into a valuable analytical signal, mostly of electrochemical or colorimetric nature. The recognition elements include a variety of substances, including proteins, nucleic acids, and others.[54] Considering its exciting potential for the development of prognostic, diagnostic, and therapeutic intervention strategies, including theranostics – such as patient prescreening and treatment monitoring, nanosensing technologies have started to acquire traction in the field of medical science. Because nanosensing technology uses smaller platforms, it can detect biomolecular signals with greater accuracy and sensitivity.[55] Although miniaturization and digitization of all parts of nanosensing POC platforms appear clinically easy and consistent, the technical characteristics of the POC platforms are a significant problem. The goal of POC approaches is to provide user-friendly biosensing platforms for detecting molecular signatures in biological fluids such as blood or urine, refrain from the need for pretreatment, and with high precision, selectivity, and accuracy. Furthermore, multiplexing or concurrent detection of several bio-analytes inside the same sample may aid in creating complete informative diagnostics.[56] Nanobiosensors can be classed according to their signal transduction method and bio-recognition components, such as electrochemical nanobiosensors, mass-sensitive nanobiosensors, calorimetric nanobiosensors, and optical nanobiosensors. Using a combination of techniques such as absorption, luminescence (fluorescence, phosphorescence, and fluorescence resonance energy transfer), Raman spectroscopy, refraction, and dispersion, optical nanobiosensors enable combinatorial detection of analytes and in vivo imaging. Unique features such as energy, polarization, amplitude, decay time, and/or phase are detected by the spectroscopic techniques. Recent data from several papers indicate that the identification of cancer biomarkers may be accomplished with success using optical nanobiosensors. At present, under investigation as nanoparticulate components for optical biosensor production consist of carbon nanodots, metal- and silica-based nanomaterials, magnetic nanostructures, and QDs.[57]
HARNESSING QDS-BASED IMAGING FOR ADVANCED BIOMARKER ASSESSMENT IN BC
The exciting subject of quantum imaging studies how to apply the ideas of quantum mechanics to image technology. Quantum imaging uses the special abilities of quantum mechanics, especially combination and entanglement, to accomplish objectives that are not feasible with conventional approaches, in contrast to conventional imaging techniques, which utilize traditional physics. Quantum entanglement, in which multiple particles become linked so that, regardless of their distance from one another, the position of one particle instantly influences the behavior of the next, is one of the fundamental ideas of quantum imaging.[58] This phenomenon can result in the creation of quantum-entangled phases of light, which have several imaging uses. Imaging-based QD sensors are cutting-edge devices with enormous promise for accurate and timely diagnosis of BC. These nanoscale semiconductor particles are perfect for biological imaging applications due to their distinctive optical and electrical characteristics, which include controllable fluorescence emission, a high quantum yield, and remarkable photostability.[59] In the context of BC detection, QDs offer several advantages over traditional imaging agents, such as organic dyes and fluorescent proteins, including brighter and more stable fluorescence signals, multiplexing capabilities for simultaneous detection of multiple biomarkers, and enhanced tissue penetration for deeper imaging depths. By conjugating QDs with specific targeting ligands, such as antibodies or peptides that recognize BC biomarkers such as human epidermal growth factor receptor 2 (HER2), ER, and progesterone receptor, researchers can design highly sensitive and selective imaging probes capable of pinpointing cancerous lesions with unprecedented precision.[60] Moreover, QDs imaging-based sensors can facilitate real-time monitoring of dynamic biological processes in living cells and tissues, enabling researchers to gain deeper insights into the molecular mechanisms underlying BC progression and metastasis. As this technology continues to evolve, it holds promise for revolutionizing BC diagnosis and personalized medicine, ushering in a new era of more effective and tailored approaches to patient care. In addition, QDs’ broad excitation spectrum and narrow emission make them advantageous for multiplexed imaging over a single excitation spectrum where the targeted signals do not interfere with one another. Due to their small size, QD particles may act as a single unit, with each of their constituent atom’s excitation and emission of light occurring concurrently to provide an effective fluorescence signal having a high signal strength.[61] QDs have been coated with various kinds of amphiphilic polymers for tumor applications to increase their water solubility and biocompatibility. Chemically, active groups such as carboxyl, hydroxyl, and thiol groups are frequently present in these amphiphilic polymers. These groups can pair with transformed functional agents including streptavidin, drugs, small molecules, DNA, and antibodies. The altered QDs may be applied to tumor research, including biomarker identification, tumor imaging, demonstrating tumor heterogeneity, and diagnosing, treating, and tracking cancer.
QD-based imaging techniques
Targeted imaging of tumor biomarkers
QDs can form conjugates with antibodies, peptides, or other ligands that possess a particular affinity for BC biomarkers, including HER2, ERs, and other surface proteins. This focused strategy enables accurate identification and imaging of malignant tissues. QDs, when combined with anti-HER2 antibodies, can specifically attach to BC cells that express HER2, allowing for their identification with great accuracy and sensitivity.[62]
In vivo tumor imaging
QDs have the potential to be used for seeing BC tumors in living organisms. Researchers can observe and follow the distribution of QD conjugates inside the body by injecting them into the circulation. Near-infrared QDs are very advantageous for in vivo imaging due to their ability to penetrate tissues extensively and exhibit low interference from biological autofluorescence. This enables the production of precise and comprehensive images of tumors and areas of metastasis.[63]
Sentinel lymph node mapping
Sentinel lymph node mapping is essential in BC surgery to determine the degree of cancer metastasis. QDs have the capability to serve as fluorescent markers for the purpose of identifying sentinel lymph nodes. QDs, when injected in close proximity to the tumor, migrate to the sentinel lymph nodes, making them detectable using fluorescence imaging. This method assists surgeons in precisely identifying and extracting lymph nodes that are impacted, hence enhancing surgical results and minimizing the need for large lymph node dissections.[64]
While QDs imaging-based sensors hold significant promise for BC detection, research in this field is ongoing and evolving rapidly. In a work by Xiao et al., the researchers found site-specific motifs that were difficult to identify using fluorescence in situ hybridization techniques along with HER-2 receptors expressed at low levels. According to research, a novel potential technique for Ki67 evaluation was made possible by QD-based immunofluorescent imaging. Ki67 value was found to be an independent predictor of prognosis in patients with HER2-positive (non-luminal) BC, particularly those without lymph nodes.[65] In this another work, they evaluate the presence of HER2 in BC using conjugates of QDs. This is a clinical use of QDs for BC molecular detection. With the use of this QDs–immunohistochemistry (IHC) analysis technique, the levels of HER2 in BC were found in an easy, digitized, quantitative, and sensitive manner. The QDs-based technique is far more specific, accurate, and cost-effective than traditional IHC, especially in situations of IHC (2þ). Furthermore, the outcomes that this approach finds may be better suited for choosing BC’s molecular-focused therapy as the study was carried out in 94 clinical samples of BC.[66] The QDs-based analytical spectral analysis was used in a study to examine the prognostic significance of estimated glomerular filtration rate (EGFR) in BC. The results indicate that QDs-immunohistochemistry performed equally to immunohistochemistry in terms of EGFR detection in BC specimens. In those with HER2-positive patients and lymph node-positive BC, the EGFR region has a poor prognostic value, according to quantitative analysis using QDs-based method.[67] In one study, researchers examined BC heterogeneity by evaluating the QD-IHC for HER2 and ER imaging. This method successfully combines quantifiable imaging of HER2 and ER expression of proteins with morphological observation. With QDIHC, the BC heterogeneity may be seen more sensitively and vividly. Moreover, concurrent imaging of ER and HER2 may provide light on how these two molecules interacted as BC heterogeneity evolved. Consequently, this method offers fresh perspectives on the variability of BC.[68] The use of QDs in cancer studies is obviously on the rise, and these particles can be extremely useful for bio-imaging and cancer diagnostics [Table 2].[69-88] A perfect clinical scenario would involve a case in which QDs-based probes might be used to detect primary tumors as well as metastatic tumors early and effectively without requiring surgery or invasive treatment.
| S. No. | QDs used | Target imaging cells | Analysis | Findings | References | |
|---|---|---|---|---|---|---|
| 1. | Gold quantum clusters | MAPK and PI3K– AKT | In vivo | A NIR nanoprobe with a significant Stokes shift was used with a smartphone featuring special optics for easier BC diagnosis and image-guided excision in vivo for BC. | [69] | |
| 2. | QD antiHER2-antibody bioconjugate |
HepG2, SK-BR-3, and MCF7 |
In vitro | The QDs antiHER2-antibody conjugates were successful in precisely localizing HER2 receptors on SK-BR-3 cells through NIR light emission. |
[70] | |
| 3. | Lipid-coated red fluorescent carbon dots | L929 and 4T1 cells | In vitro | When exposed to laser light, LRCDs can effectively kill 4T1 cells by generating heat and toxins. The CAM assay confirmed that LRCDs with NIR radiation had strong photothermal effects. | [71] | |
| 4. | Nitrogen-doped graphene QDs | MDA-MB-231 | In vitro and in vivo | Nanoparticles incubated with MDA-MB-231 cells can promote fluorescence imaging at different wavelengths. | [72] | |
| 6. | Chitosan/carbon QDs/Fe2O3 | MCF-7 | In vitro | CS/CQDs/Fe2O3 encapsulated curcumin exhibits cytotoxicity against MCF-7 cells, indicating its potential use in targeted drug delivery and bioimaging. | [73] | |
| 7. | FeSe QDs | HER2-overexpressed MCF7 cells | In vitro and in vivo | Imaging was done up to 500 µm from the skin using a femtosecond laser at 800 nm. FeSe QDs may be used in multiphoton cancer imaging. | [74] | |
| 8. | Layered gelatin/chondroitin QDs | MCF-7 and MDA-MB-231 | In vitro and in vivo | Three multifunctional nanoplatforms (CS-NCs, G-CS-NCs, and G-QDs-CS-NCs) were developed for the co-delivery of CXB and RAP in BC therapy. G-QDs-CS-NCs could be used as a theranostics tool for cancer imaging and therapy. | [75] | |
| 9. | Carbon dots | MCF-7 cells | In vitro | Carbon dots emit green light in cancer cells, allowing for targeted detection. | [76] | |
| 10. | CuInS2/ZnS QDs | Ki-67 Biomarker | In vitro | CuInS2/ZnS QDs labeled bioprobe - QD-Ki-67 is non-toxic and maintains the optical properties of unadorned QDs. | [77] | |
| 11. | QD-lipid nanocarriers | MDA-MB-231 | In vitro and in vivo | EGFR aptamer-guided lipid carriers may deliver RNA interference and fluorescence imaging to TNBCs. | [78] | |
| 12. | CdSe/ZnS QDs | HER2 | In vivo | Purified QD-HER2-Ab probes can accurately target tumors and detect HER2, minimizing background signal and toxicity in vivo. This makes it a convenient tool for in vivo tumor imaging. | [79] | |
| 13. | CdTe QDs | MCF-7 | In vitro | ATP in lysosomes caused QDs to be released from GNPs, resulting in fluorescence. MCF-7 cells internalized the DANP complex. | [80] | |
| 14. | Mn: ZnS QDs | MCF-7 and MDA-MB-231 | In vitro | Folic acid enhances the cellular uptake of chitosan-encapsulated QDs, making them a promising option for targeted drug delivery and cellular imaging. | [81] | |
| 15. | CdSe/ZnS QDs | In vitro | The new nanoformulation-induced cell apoptosis and showed 55% cytotoxicity. Fluorescence imaging confirmed cellular uptake, making it a promising candidate for cancer theranostics applications. | [82] | ||
| 16. | GO-PEI-GQDs | MDA-MB-231 | In vitro | GO-PEI-GQDs show strong photothermal and cytotoxic activities on cancer cells, and their composite materials can improve therapeutic systems in theranostics. | [83] | |
| 17. | QD-based IIQAS | Topoisomerase 2 alpha | Immunohistochemistry | QD-IIQAS found a high correlation (r=0.79) and agreement(κ=0.763) of TOP2A expression in TNBC, with similar antigen localization. | [84] | |
| 18. | QD | HER2 | In vivo | QD probes were used to visualize tumors through in vivo imaging. QD-4D5scFv produced a stronger fluorescent signal in tumors compared to QD-PEG. | [85] | |
| 19. | Polymer-encapsulated and bioconjugate QD | MDAMB-231 | In vivo | In vivoimaging shows that active targeting with QD probes is faster and more effective than passive targeting for tumor locations. Using QD probes with wavelength-resolved imaging enables sensitive tricolor imaging of cancer cells in vivo animals. | [86] | |
| 20. | αvβ3-integrin-targeted quantum-dots-loaded liposome–microbubble(iRGD-QDLM) | 4T1 BC cells | In vitroand in vivo | A new liposome-microbubble complex for tumor imaging uses QDs and iRGD lipopeptide to target αvβ3-integrin with potential for clinical translation. It could be used for ultrasound molecular imaging to detect metastasis-related biomarkers. | [87] | |
| 21. | Mg/N doped-carbon QDs | 4T1 and MCF-7 | In vitroand in vivo | Compared to the free drugs, the complex entered 4T1 and MCF-7 cells at a higher rate during cell imaging, indicating that the synthesized complex was functioning as intended. | [88] | |
MAPK: Mitogen-activated protein kinase, PI3K–AKT: Phosphoinositide-3-kinase–protein kinase B/Akt, HepG2: Hepatoblastoma cell line, MCF7: Michigan Cancer Foundation-7, HER2: Human epidermal growth factor receptor 2, GNPs: Gold nanoparticles, GO-PEI-GQDs: Graphene oxide-polyethylene imine-Graphene quantum dots, QDs: Quantum dots, NIR: Near-infrared, QD-IIQAS: Quantum dot-immunofluorescent imaging and quantitative analytical system, LRCDs: Lipid-coated red fluorescent carbon dots, CAM: Chick chorioallantoic membrane, TNBCs: Triple-negative breast cancer, DANP: Double aptamer-nanoparticle conjugates-based, MDA-MB: M.D. Anderson Metastasis Breast cancer, CS: Chitosan.
TRANSLATIONAL VALUE FROM ARTIFICIAL INTELLIGENCE (AI) PERSPECTIVE
The evaluation of quantum imaging data for the purpose of detecting BC is being revolutionized by AI, which is a key factor in this transformation. AI has several uses, including improving patient outcomes, streamlining procedures, and increasing diagnostic accuracy. Because the obtained data are so complex, quantum imaging techniques pose special problems that frequently require sophisticated computational methods for interpretation. AI systems are particularly good in this area since they can first improve images by enhancing quantum images to bring out details that are important for early cancer identification.[89] AI uses complex algorithms to make it easier to extract complex aspects from quantum pictures, such as subtle textures, spatial patterns, and intensity fluctuations, enabling more thorough study than is possible for humans. Furthermore, deep learning neural networks and other AI-driven classification algorithms are essential for classifying quantum visuals into clinically relevant groups and accurately identifying benign and malignant cells.[90] AI also supports personalized medicine by combining data from quantum imaging with other patient characteristics, including genetic profiles and medical histories, to enable customized risk assessment and treatment plans. AI has significant promise for real-time diagnostics in quantum imaging, providing quick analysis during imaging operations, accelerating decision-making, and maybe cutting down on diagnosis time. Furthermore, AI ensures constant diagnostic performance by closely examining quantum imaging systems for accuracy and dependability. This promotes ongoing quality control. AI drives research and development efforts in quantum imaging, boosting innovation and discovery in this emerging sector, even beyond clinical applications.[91] To achieve its disruptive potential responsibly, AI in healthcare must be implemented with strict validation, ethical concerns, and smooth integration into clinical procedures. Fundamentally, AI becomes an invaluable ally in utilizing quantum imaging to diagnose BC early and accurately, hence changing the diagnostic medical landscape. Another aspect of AI, that is, machine learning (ML) and applying ML techniques built around quantum computing principles has generated a new discipline termed quantum machine learning (QML) that has greatly enhanced traditional ML applications. To improve the rapidity and efficacy of ML in comparison to traditional computer equivalents, QML investigates the design and implementation of quantum software. QML implements quantum algorithms, which may outperform traditional machine learning techniques for particular tasks. Several QML methods have been suggested, including quantum reinforcement learning (QRL). Through the use of quantum computing, QRL raises the performance bar for traditional RL algorithms. Large volumes of data can be processed and analyzed by quantum computers at once, giving them the ability to explore a wider state space and find the best solutions more quickly. In medical applications, where prompt and accurate judgments are essential, QRL may greatly accelerate learning and enhance decision-making accuracy. When it comes to the detection of BC, QRL may be used to create models that integrate many kinds of data, such as genetic, imaging, and biomarker information, to streamline the diagnostic process. With each new set of data, these models may learn and become better over time, producing more precise and individualized diagnostic suggestions.[92] Quantum principal component analysis (PCA), A statistical method called PCA, is used to minimize the dimensionality of huge datasets while retaining the most significant variation in the data. To capture the most variance, principal components – a new collection of orthogonal variables – are created from the data using PCA. To detect patterns and correlations that can be suggestive of cancer, PCA can be used to evaluate complicated datasets including genetic profiles, medical imaging, and biomarker levels in the identification of BC.[93] Quantum Support Vector Machines (QSVM), compared to conventional support vector machines, quantum support vector machines handle complicated optimization problems more quickly using the capabilities of quantum computing. Because quantum computers can do several operations at once, or quantum parallelism, they can handle and analyze enormous datasets at previously unheard of rates. Quantum support vector machines (QSVMs) use quantum algorithms to determine the best hyperplane for classification, which might result in quicker and more precise diagnostic results. QSVMs outperform traditional algorithms in the analysis of high-dimensional data related to BC detection, including genetic profiles and imaging images. This improved aptitude makes it possible to see minute correlations and patterns that could point to the existence of cancer, even at an early stage. QSVMs may decrease false positives and negatives, increasing the accuracy of diagnostic models and facilitating more dependable screening and early diagnosis. QRL operates very well in quantum simulators (QS).[94,95] Quantum computing may benefit from the strength of quantum entanglement, quantum superposition, and quantum coherence. These essential quantum phenomena open the door for a unique information processing methodology that might surpass traditional approaches for the detection of BC.
CHALLENGES AND FUTURE DIRECTIONS
The potential of quantum imaging-based sensors in clinical settings is indisputable. However, certain technical difficulties need to be addressed before their widespread adoption. These include scalability, cost-effectiveness, and integration with existing imaging infrastructure. In addition, standardization and regulatory approval are prerequisites to ensure the safety and efficacy of quantum imaging technologies in therapeutic environments. More research is required to refine imaging procedures, validate diagnosis accuracy, and assess long-term outcomes. Despite these challenges, quantum imaging-based sensors for BC detection hold significant promise for the future. Advancements in computing algorithms, materials science, and quantum technologies will enhance imaging capabilities and expand their range of applications. Collaboration between physicists, engineers, physicians, and industry stakeholders can facilitate innovation and promote the translation of research into clinical practice. Ultimately, by enabling earlier detection, more precise staging, and personalized treatment plans, quantum imaging-based sensors are poised to revolutionize BC detection and improve patient outcomes.
CONCLUSION
As QDs imaging-based sensors continue to mature, their translation from bench to bedside holds immense promise for improving clinical outcomes in BC patients. By facilitating early detection of tumors, monitoring treatment responses, and guiding surgical interventions, QD-based imaging technologies can empower clinicians with actionable insights for personalized patient management. Moreover, their potential for non-invasive imaging and image-guided therapies offers new avenues for minimally invasive cancer interventions, minimizing patient discomfort and improving treatment efficacy. With its previously unheard-of accuracy, precision, and non-invasiveness, quantum imaging-based sensors mark a paradigm leap in BC diagnosis. These cutting-edge devices have the power to change healthcare practice and enhance patient outcomes by utilizing the ideas of quantum mechanics. Even if there are still obstacles to overcome, continuous research and development activities are opening the door for the regular detection and diagnosis of BC using quantum imaging-based sensors. With the ongoing pursuit of fully realizing the endless possibilities of quantum technology, the prospects for early diagnosis of BC have become increasingly promising.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that no artificial intelligence (AI)-assisted technology was used to assist in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
PR expresses her gratitude to the Indian Council of Medical Research (ICMR), Department of Health Research (DHR), Ministry of Health and Family Welfare, and the Council of Scientific Research (CSIR), Government of India, New Delhi, for providing financial support in the form of a Senior Research Fellowship (SRF).
References
- Socioeconomic variations of breast cancer treatment and discontinuation: A study from a public tertiary cancer hospital in Mumbai, India. BMC Womens Health. 2023;23:113.
- [CrossRef] [PubMed] [Google Scholar]
- Household air pollution. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health [Last accessed on 2024 Jun 25]
- [Google Scholar]
- Critical review on emerging health effects associated with the indoor air quality and its sustainable management. Sci Total Environ. 2023;872:162163.
- [CrossRef] [PubMed] [Google Scholar]
- Exposure to ultrafine particulate matter induces NF-κβ mediated epigenetic modifications. Environ Pollut. 2019;252:39-50.
- [CrossRef] [PubMed] [Google Scholar]
- A review of diseases associated with household air pollution due to the use of biomass fuels. J Hazard Mater. 2011;192:425-31.
- [CrossRef] [PubMed] [Google Scholar]
- Breast cancer early detection: A phased approach to implementation. Cancer. 2020;126:2379-93.
- [CrossRef] [PubMed] [Google Scholar]
- MRI for breast cancer screening, diagnosis, and treatment. Lancet. 2011;378:1804-11.
- [CrossRef] [PubMed] [Google Scholar]
- A multiparametric [18 F] FDG PET/MRI diagnostic model including imaging biomarkers of the tumor and contralateral healthy breast tissue aids breast cancer diagnosis. Eur J Nucl Med Mol Imaging. 2019;46:1878-88.
- [CrossRef] [PubMed] [Google Scholar]
- Nanophotonic biosensors as point-of-care tools for preventive health interventions. Nanomedicine (Lond). 2020;15:1541-4.
- [CrossRef] [PubMed] [Google Scholar]
- Nanobiosensors: Point-of-care approaches for cancer diagnostics. Biosens Bioelectron. 2019;130:147-65.
- [CrossRef] [PubMed] [Google Scholar]
- Bioanalytical applications of graphene quantum dots for circulating cell-free nucleic acids: A review. ACS Omega. 2022;7:39586-602.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine disruptors from the environment affecting breast cancer. Oncol Lett. 2020;20:19-32.
- [CrossRef] [PubMed] [Google Scholar]
- A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol. 2015;40:241-58.
- [CrossRef] [PubMed] [Google Scholar]
- Ozone-induced DNA damage: A Pandora's box of oxidatively modified DNA bases. Chem Res Toxicol. 2021;34:80-90.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiologic evidence of exposure to polycyclic aromatic hydrocarbons and breast cancer: A systematic review and meta-analysis. Chemosphere. 2022;290:133237.
- [CrossRef] [PubMed] [Google Scholar]
- Outdoor ambient air pollution and breast cancer survival among California participants of the Multiethnic Cohort Study. Environ Int. 2022;161:107088.
- [CrossRef] [PubMed] [Google Scholar]
- Particulate matter and traffic-related exposures in relation to breast cancer survival. Cancer Epidemiol Biomarkers Prev. 2019;28:751-9.
- [CrossRef] [PubMed] [Google Scholar]
- Association between ambient air pollution and breast cancer risk: The multiethnic cohort study. Int J Cancer. 2020;146:699-711.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated blood pressure and household solid fuel use in premenopausal women: Analysis of 12 Demographic and Health Surveys (DHS) from 10 countries. Environ Res. 2018;160:499-505.
- [CrossRef] [PubMed] [Google Scholar]
- Radon exposure-therapeutic effect and cancer risk. Int J Mol Sci. 2020;22:316.
- [CrossRef] [PubMed] [Google Scholar]
- Cell-free circulating miRNAs-lncRNAsmRNAs as predictive markers for breast cancer risk assessment in women exposed to indoor air pollution. Case Stud Chem Environ Eng. 2022;6:100267.
- [CrossRef] [Google Scholar]
- Environmental exposures and breast cancer risk in the context of underlying susceptibility: A systematic review of the epidemiological literature. Environ Res. 2020;187:109346.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence of biomass smoke exposure as a causative factor for the development of COPD. Toxics. 2017;5:36.
- [CrossRef] [PubMed] [Google Scholar]
- Indoor air pollution exposure from use of indoor stoves and fireplaces in association with breast cancer: A case-control study. Environ Health. 2014;13:108.
- [CrossRef] [PubMed] [Google Scholar]
- Exposure to multiple sources of polycyclic aromatic hydrocarbons and breast cancer incidence. Environ Int. 2016;89:185-92.
- [CrossRef] [PubMed] [Google Scholar]
- Indoor wood-burning stove and fireplace use and breast cancer in a prospective cohort study. Environ Health Perspect. 2017;125:077011.
- [CrossRef] [PubMed] [Google Scholar]
- Women's occupational exposure to polycyclic aromatic hydrocarbons and risk of breast cancer. Occup Environ Med. 2019;76:22-9.
- [CrossRef] [PubMed] [Google Scholar]
- Solid fuel use for heating and risks of breast and cervical cancer mortality in China. Environ Res. 2020;186:109578.
- [CrossRef] [PubMed] [Google Scholar]
- Organic extract of indoor dust induces estrogen-like effects in human breast cancer cells. Sci Total Environ. 2020;726:138505.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic low-dose exposure to xenoestrogen ambient air pollutants and breast cancer risk: XENAIR protocol for a case-control study nested within the French E3N cohort. JMIR Res Protoc. 2020;9:e15167.
- [CrossRef] [PubMed] [Google Scholar]
- Household air pollution from solid cooking fuel combustion and female breast cancer. Front Public Health. 2021;9:677851.
- [CrossRef] [PubMed] [Google Scholar]
- Residential proximity to dioxin emissions and risk of breast cancer in the sister study cohort. Environ Res. 2023;222:115297.
- [CrossRef] [PubMed] [Google Scholar]
- Exposure to polycyclic aromatic hydrocarbons during pregnancy and breast tissue composition in adolescent daughters and their mothers: A prospective cohort study. Breast Cancer Res. 2022;24:47.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of disease attributable to secondhand smoke exposure: A systematic review. Prev Med. 2019;129:105833.
- [CrossRef] [PubMed] [Google Scholar]
- Linking exposure assessment science with policy objectives for environmental justice and breast cancer advocacy: The northern California household exposure study. Am J Public Health. 2009;99(Suppl 3):S600-9.
- [CrossRef] [PubMed] [Google Scholar]
- Antimüllerian hormone in relation to tobacco and marijuana use and sources of indoor heating/cooking. Fertil Steril. 2016;106:723-30.
- [CrossRef] [PubMed] [Google Scholar]
- Exposure to household tobacco smoke and risk of cancer morbidity and mortality: Analysis of data from the Afghanistan Demographic and Health Survey 2015. Prev Med. 2019;123:217-24.
- [CrossRef] [PubMed] [Google Scholar]
- Household electromagnetic fields and breast cancer in elderly women. In Vivo. 2005;19:563-6.
- [Google Scholar]
- Organophosphorus flame retardants and phthalate esters in indoor dust from different microenvironments: Bioaccessibility and risk assessment. Chemosphere. 2016;150:528-35.
- [CrossRef] [PubMed] [Google Scholar]
- Organophosphate ester tri-o-cresyl phosphate interacts with estrogen receptor α in MCF-7 breast cancer cells promoting cancer growth. Toxicol Appl Pharmacol. 2020;395:114977.
- [CrossRef] [PubMed] [Google Scholar]
- Bisphenol A stimulates the epithelial mesenchymal transition of estrogen negative breast cancer cells via FOXA1 signals. Arch Biochem Biophys. 2015;585:10-6.
- [CrossRef] [PubMed] [Google Scholar]
- DNA damage in mice treated with sulfur dioxide by inhalation. Environ Mol Mutagen. 2005;46:150-5.
- [CrossRef] [PubMed] [Google Scholar]
- Sources of polycyclic aromatic hydrocarbons are associated with gene-specific promoter methylation in women with breast cancer. Environ Res. 2016;145:93-100.
- [CrossRef] [PubMed] [Google Scholar]
- Integrated mitoepigenetic signalling mechanisms associated with airborne particulate matter exposure: A cross-sectional pilot study. Atmos Pollut Res. 2022;13:101399.
- [CrossRef] [Google Scholar]
- Prenatal exposure to environmental pro-oxidants induces mitochondria-mediated epigenetic changes: A cross-sectional pilot study. Environ Sci Pollut Res. 2022;29:74133-49.
- [CrossRef] [PubMed] [Google Scholar]
- Ambient particulate matter and microRNAs in extracellular vesicles: A pilot study of older individuals. Part Fibre Toxicol. 2015;13:1-13.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative profiling of epigenetic modifications among individuals living in different high and low air pollution zones: A pilot study from India. Environ Adv. 2021;4:100052.
- [CrossRef] [Google Scholar]
- Ambient particulate air pollution and microRNAs in elderly men. Epidemiology. 2014;25:68-78.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term exposure to ambient air pollution and incidence of postmenopausal breast cancer in 15 European cohorts within the ESCAPE project. Environ Health Perspect. 2017;125:107005.
- [CrossRef] [PubMed] [Google Scholar]
- Environmental tobacco smoke and cancer risk, a prospective cohort study in a Chinese population. Environ Res. 2020;191:110015.
- [CrossRef] [PubMed] [Google Scholar]
- Air pollution, particulate matter composition and methylation-based biologic age. Environ Int. 2019;132:105071.
- [CrossRef] [PubMed] [Google Scholar]
- Photonics and optoelectronics using nano-structured hybrid perovskite media and their optical cavities. Phys Rep. 2019;795:1-51.
- [CrossRef] [Google Scholar]
- Current trends of nanobiosensors for point-of-care diagnostics. J Anal Methods Chem. 2019;2019:2179718.
- [CrossRef] [PubMed] [Google Scholar]
- Progress of infrared guided-wave nanophotonic sensors and devices. Nano Converg. 2020;7:12.
- [CrossRef] [PubMed] [Google Scholar]
- MIP-based sensors: Promising new tools for cancer biomarker determination. Sensors. 2017;17:718.
- [CrossRef] [PubMed] [Google Scholar]
- Perspectives for applications of quantum imaging. Laser Photonics Rev. 2019;13:1900097.
- [CrossRef] [Google Scholar]
- Quantum dots in imaging, drug delivery and sensor applications. Int J Nanomedicine. 2017;12:5421-31.
- [CrossRef] [PubMed] [Google Scholar]
- Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem Soc Rev. 2015;44:4792-834.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor diagnosis using carbon-based quantum dots: Detection based on the hallmarks of cancer. Bioact Mater. 2024;33:174-222.
- [CrossRef] [PubMed] [Google Scholar]
- Peptides targeting HER2-positive breast cancer cells and applications in tumor imaging and delivery of chemotherapeutics. Nanomaterials (Basel). 2023;13:2476.
- [CrossRef] [PubMed] [Google Scholar]
- Near-infrared-II quantum dots for in vivo imaging and cancer therapy. Small. 2022;18:e2104567.
- [CrossRef] [PubMed] [Google Scholar]
- Advances and perspectives in nanoprobes for noninvasive lymph node mapping. In: Nanomedicine (Lond). Vol 10. 2015. p. :1019-36. Erratum in: Nanomedicine (Lond) 2015;10:1992
- [CrossRef] [PubMed] [Google Scholar]
- Quantitation of HER2 and telomerase biomarkers in solid tumors with IgY antibodies and nanocrystal detection. Int J Cancer. 2008;122:2178-86.
- [CrossRef] [PubMed] [Google Scholar]
- Quantum dots-based immunofluorescence technology for the quantitative determination of HER2 expression in breast cancer. Biomaterials. 2009;30:2912-8.
- [CrossRef] [PubMed] [Google Scholar]
- Quantum dot-based quantitative immunofluorescence detection and spectrum analysis of epidermal growth factor receptor in breast cancer tissue arrays. Int J Nanomedicine. 2011;6:2265-73.
- [CrossRef] [PubMed] [Google Scholar]
- Quantum-dot-based immunofluorescent imaging of HER2 and ER provides new insights into breast cancer heterogeneity. Nanotechnology. 2010;21:095101.
- [CrossRef] [PubMed] [Google Scholar]
- Gold/alpha-lactalbumin nanoprobes for the imaging and treatment of breast cancer. Nat Biomed Eng. 2020;4:686-703.
- [CrossRef] [PubMed] [Google Scholar]
- Near-infrared quantum dots for HER2 localization and imaging of cancer cells. Int J Nanomedicine. 2014;9:1323-37.
- [CrossRef] [PubMed] [Google Scholar]
- Lipid-coated red fluorescent carbon dots for imaging and synergistic phototherapy in breast cancer. Photodiagnosis Photodyn Ther. 2023;41:103314.
- [CrossRef] [PubMed] [Google Scholar]
- Organotin (IV)-decorated graphene quantum dots as dual platform for molecular imaging and treatment of triple negative breast cancer. Chem Eur J. 2023;29:202301845.
- [CrossRef] [PubMed] [Google Scholar]
- Synthesis and characterization of chitosan/carbon quantum dots/Fe2O3 nanocomposite comprising curcumin for targeted drug delivery in breast cancer therapy. Int J Biol Macromol. 2023;249:125788.
- [CrossRef] [PubMed] [Google Scholar]
- FeSe quantum dots for in vivo multiphoton biomedical imaging. Sci Adv. 2019;5:0044.
- [CrossRef] [PubMed] [Google Scholar]
- Layer-by-layer gelatin/chondroitin quantum dots-based nanotheranostics: Combined rapamycin/celecoxib delivery and cancer imaging. Nanomedicine. 2018;13:1707-30.
- [CrossRef] [PubMed] [Google Scholar]
- Fabrication of FA/HA-functionalized carbon dots for human breast cancer cell targeted imaging. Photodiagnosis Photodyn Ther. 2022;40:103099.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting breast cancer cells with a CuInS2/ZnS quantum dot-labeled Ki-67 bioprobe. Oncol Lett. 2018;15:2471-6.
- [CrossRef] [Google Scholar]
- Anti-EGF receptor aptamer-guided co-delivery of anti-cancer siRNAs and quantum dots for theranostics of triple-negative breast cancer. Theranostics. 2019;9:837.
- [CrossRef] [PubMed] [Google Scholar]
- Purified fluorescent nanohybrids based on quantum dotHER2-antibody for breast tumor target imaging. Talanta. 2023;260:124560.
- [CrossRef] [PubMed] [Google Scholar]
- Targeted imaging of breast cancer cells using two different kinds of aptamers-functionalized nanoparticles. Eur J Pharm Sci. 2019;134:60-8.
- [CrossRef] [PubMed] [Google Scholar]
- Folic acid targeted Mn: ZnS quantum dots for theranostic applications of cancer cell imaging and therapy. Int J Nanomedicine. 2016;11:413-28.
- [CrossRef] [PubMed] [Google Scholar]
- A new theranostic pH-responsive niosome formulation for doxorubicin delivery and bio-imaging against breast cancer. Int J Pharm. 2023;637:122845.
- [CrossRef] [PubMed] [Google Scholar]
- Preparation of graphene oxide-graphene quantum dots hybrid and its application in cancer theranostics. Mater Sci Eng C. 2019;103:109774.
- [CrossRef] [PubMed] [Google Scholar]
- Quantum dot-based immunofluorescent imaging and quantitative detection of TOP2A and prognostic value in triple-negative breast cancer. Int J Nanomedicine. 2016;11:5519-29.
- [CrossRef] [PubMed] [Google Scholar]
- Passive and active targeting of quantum dots for whole-body fluorescence imaging of breast cancer xenografts. J Biophotonics. 2012;5:860-7.
- [CrossRef] [PubMed] [Google Scholar]
- In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969-76.
- [CrossRef] [PubMed] [Google Scholar]
- Dual-modality molecular imaging of tumor via quantum dots-liposome-microbubble complexes. Pharmaceutics. 2022;14:2510.
- [CrossRef] [PubMed] [Google Scholar]
- Dual targeting of Mg/N doped-carbon quantum dots with folic and hyaluronic acid for targeted drug delivery and cell imaging. Biomed Pharmacother. 2023;164:114971.
- [CrossRef] [PubMed] [Google Scholar]
- Quantum machine learning: A review and case studies. Entropy. 2023;25:287.
- [CrossRef] [PubMed] [Google Scholar]
- Quantum machine learning in medical image analysis: A survey. Neurocomputing. 2023;525:42-53.
- [CrossRef] [Google Scholar]
- Machine learning in the quantum realm: The state-of-the-art, challenges, and future vision. Expert Syst Appl. 2022;194:116512.
- [CrossRef] [Google Scholar]
- Breast cancer diagnosis using support vector machine optimized by improved quantum inspired grey wolf optimization. Sci Rep. 2024;14:10714.
- [CrossRef] [PubMed] [Google Scholar]
- Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2014;13:8-17.
- [CrossRef] [PubMed] [Google Scholar]
- Quantum computing in molecular magnets. Nature. 2001;410:789-93.
- [CrossRef] [PubMed] [Google Scholar]
- Quantum transfer learning for breast cancer detection. Quantum Mach Intell. 2022;4:5.
- [CrossRef] [PubMed] [Google Scholar]






